Your Fluorine ion symbol and charge images are ready. Fluorine ion symbol and charge are a topic that is being searched for and liked by netizens today. You can Get the Fluorine ion symbol and charge files here. Find and Download all free images.
If you’re searching for fluorine ion symbol and charge pictures information connected with to the fluorine ion symbol and charge interest, you have visit the right blog. Our website always provides you with suggestions for seeking the maximum quality video and picture content, please kindly search and locate more informative video articles and images that fit your interests.
Fluorine Ion Symbol And Charge. Science Chemistry Chemistry questions and answers The element fluorine forms an with the charge The symbol for this ion is and the name is ion. Fluoride ion F- CID 28179 - structure chemical names physical and chemical properties classification patents literature biological activities safety. Fluorine is the lightest halogen and exists as a highly toxic pale yellow diatomic gas at standard conditions. These have no charge.
 Fluorine Production And Use Britannica From britannica.com
Fluorine Production And Use Britannica From britannica.com
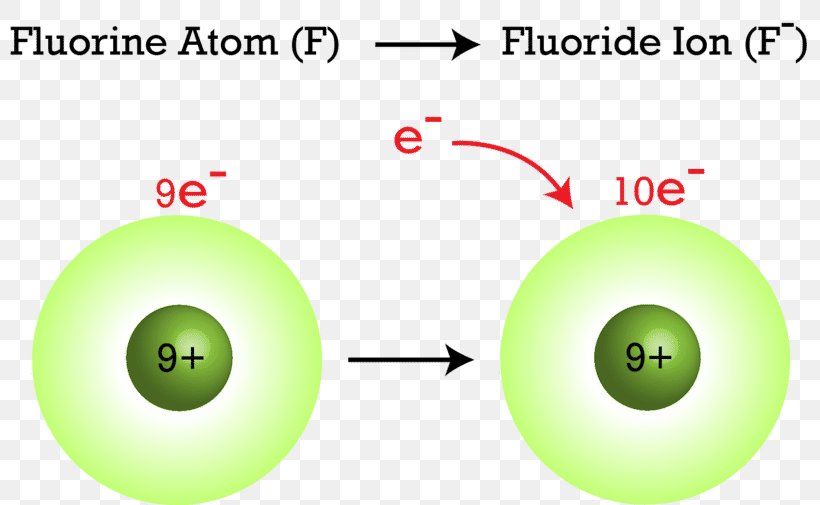
If a fluorine atom gains an electron. As the most electronegative element it is extremely reactive. Literature and Language. If atoms gain electrons they become negative ions or anions. Click to see full answer. What is the formula for ammonium carbonate.
In this post we also have variety of handy Resume example about Fluorine Ion Charge with a lot of.
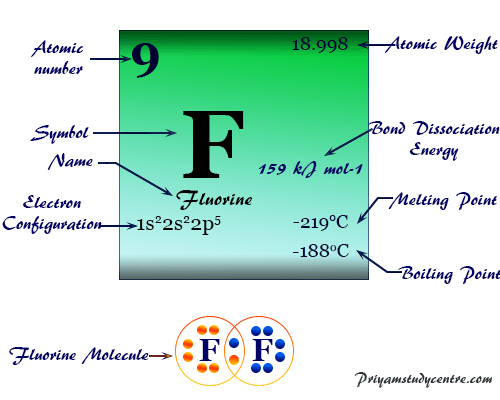
Fluorine is a chemical element with the symbol F and atomic number 9. Fluorine is element no 9 on the Periodic Table. The chemical symbol for Fluorine is F. Fluoride has an additional electron and therefore would have a negative one charge. Iodine is the heaviest of the stable halogens it exists as a lustrous purple-black metallic solid at standard conditions that sublimes readily to form a violet gas Modify. Math and Arithmetic.
 Source: youtube.com
Source: youtube.com
In this post we also have variety of handy Resume example about Fluorine Ion Charge with a lot of. In this post we also have variety of handy Resume example about Fluorine Ion Charge with a lot of. Fluorine F2 CID 24524 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazards. What is the charge of fluorine ion. This site have 12 Resume models about Fluorine Ion Charge including paper sample paper example coloring page pictures coloring page sample Resume models Resume example Resume pictures and more.

It is the lightest halogen and exists at standard conditions as a highly toxic pale yellow diatomic gas. 93 rows Ionic charge. Step 1 - Write down the symbol and charge of the first word. The total electrical charge of the nucleus is therefore Ze where e elementary charge equals to 1602 x 10-19 coulombs. Among the elements fluorine ranks 24th in universal abundance and.
 Source: favpng.com
Source: favpng.com
93 rows Ionic charge. Iodine Symbol and charge Iodine is a chemical element with atomic number 53 which means there are 53 protons and 53 electrons in the atomic structure. Fluorine has no charge as this is the name of the element which by definition has no net chargeIt has 9 protons and 9 electrons. Fluorine Ion Charge is available for you to search on this website. Consider the example of fluorine see Figure below.
 Source: slideplayer.com
Source: slideplayer.com
Fluorine Ion Charge is available for you to search on this website. Fluorine has no charge as this is the name of the element which by definition has no net chargeIt has 9 protons and 9 electrons. What is the charge of fluorine ion. The symbol for the element fluorine is F. The chemical symbol for Iodine is I.

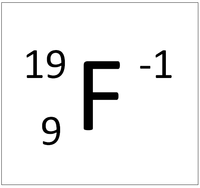
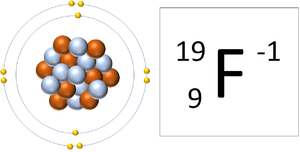
That would be 9 positive charges protons and 9 negative charges electrons. Fluorine is the lightest halogen and exists as a highly toxic pale yellow diatomic gas at standard conditions. Fluorine has no charge as this is the name of the element which by definition has no net chargeIt has 9 protons and 9 electrons. Fluoride is the negative ion of the element fluorine. The symbol for fluorine as an ion is F-.
 Source: priyamstudycentre.com
Source: priyamstudycentre.com
Step 1 - Write down the symbol and charge of the first word. 93 rows Ionic charge. If atoms gain electrons they become negative ions or anions. Fluorine F2 CID 24524 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazards. Fluoride is the negative ion of the element fluorine.
 Source: chemistrylearner.com
Source: chemistrylearner.com
The symbol for fluorine as an ion is F-. A fluorine atom has nine protons and nine electrons so it is electrically neutral. Fluorine Ion Charge is available for you to search on this website. Fluorine is a chemical element with atomic number 9 which means there are 9 protons in its nucleus. What is the charge of fluorine ion.
 Source: youtube.com
Source: youtube.com
It is the lightest halogen and exists at standard conditions as a highly toxic pale yellow diatomic gas. Fluorine is element no 9 on the Periodic Table. What is the formula for ammonium carbonate. A fluorine atom has nine protons and nine electrons so it is electrically neutral. Among the elements fluorine ranks 24th in universal abundance and.
 Source: keystagewiki.com
Source: keystagewiki.com
Result Br Step 3 - Use the minimum number of cations and anions needed to make the sum of all charges in the formula equal zero. This site have 12 Resume models about Fluorine Ion Charge including paper sample paper example coloring page pictures coloring page sample Resume models Resume example Resume pictures and more. If a fluorine atom gains an electron. The number of electrons in this ion is What is the formula for ammonium fluoride. Result Na Step 2 - Write down the symbol and charge of the second word.
 Source: youtube.com
Source: youtube.com
The number of electrons in this ion is What is the formula for ammonium fluoride. Fluorine is the lightest halogen and exists as a highly toxic pale yellow diatomic gas at standard conditions. If atoms gain electrons they become negative ions or anions. Literature and Language. The symbol for fluorine as an ion is F-.
 Source: docbrown.info
Source: docbrown.info
Any compound whether it is organic or inorganic that contains the fluoride ion is also known as a fluoride. Consider the example of fluorine see Figure below. Click to see full answer. The number of electrons in this ion is What is the formula for ammonium fluoride. As the most electronegative element it is extremely reactive.
 Source: knowledgedoor.com
Source: knowledgedoor.com
Fluorine has no charge as this is the name of the element which by definition has no net chargeIt has 9 protons and 9 electrons. Fluoride has an additional electron and therefore would have a negative one charge. Fluorine has no charge as this is the name of the element which by definition has no net chargeIt has 9 protons and 9 electrons. The symbol for the element fluorine is F. Fluoride is the negative ion of the element fluorine.
 Source: keystagewiki.com
Source: keystagewiki.com
What is the charge of fluorine ion. Fluorine has no charge as this is the name of the element which by definition has no net chargeIt has 9 protons and 9 electrons. 93 rows Ionic charge. Result Br Step 3 - Use the minimum number of cations and anions needed to make the sum of all charges in the formula equal zero. As the most electronegative element it is extremely reactive.
 Source: nagwa.com
Source: nagwa.com
Fluorine is a chemical element with the symbol F and atomic number 9. Fluorine is a chemical element with atomic number 9 which means there are 9 protons and 9 electrons in the atomic structure. Answer 1 of 5. These have no charge. If a fluorine atom gains an electron.
 Source: slideplayer.com
Source: slideplayer.com
Fluorine Ion Charge is available for you to search on this website. Otherwise fluorine is a diatomic molecule F2 As an ion it has a 2 charge so it can be written as Ca2. In this post we also have variety of handy Resume example about Fluorine Ion Charge with a lot of. Science Chemistry Chemistry questions and answers The element fluorine forms an with the charge The symbol for this ion is and the name is ion. It has only one stable isotope F-19 which means that its nucleus also contains 10 neutrons.

Fluorine F2 CID 24524 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazards. As such it has a nucleus containing 9 protons. The symbol for the element fluorine is F. The total electrical charge of the nucleus is therefore Ze where e elementary charge equals to 1602 x 10-19 coulombs. The chemical symbol for Fluorine is F.
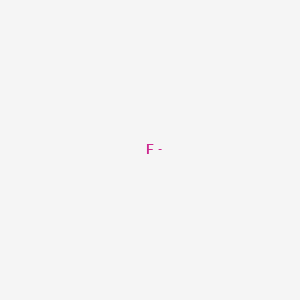
That would be 9 positive charges protons and 9 negative charges electrons. Among the elements fluorine ranks 24th in universal abundance and. What is the charge of fluorine ion. Otherwise fluorine is a diatomic molecule F2 As an ion it has a 2 charge so it can be written as Ca2. Fluorine is the lightest halogen and exists as a highly toxic pale yellow diatomic gas at standard conditions.
 Source: britannica.com
Source: britannica.com
93 rows Ionic charge. Click to see full answer. The chemical symbol for Fluorine is F. Otherwise fluorine is a diatomic molecule F2 As an ion it has a 2 charge so it can be written as Ca2. Similarly it is asked what is the charge of fluorine.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site adventageous, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title fluorine ion symbol and charge by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






