Your How to find isotope symbol images are ready. How to find isotope symbol are a topic that is being searched for and liked by netizens now. You can Get the How to find isotope symbol files here. Get all royalty-free vectors.
If you’re searching for how to find isotope symbol images information linked to the how to find isotope symbol interest, you have come to the right blog. Our site always gives you suggestions for refferencing the maximum quality video and picture content, please kindly hunt and find more informative video articles and images that match your interests.
How To Find Isotope Symbol. Click to see full answer. The mole and Avogadros number. What is its isotope notation. Each element has a standard number of neutrons that can be found by looking at a periodic table.
 Atomic Structure Relation To Nucleon Numbers Isotopes And Isotopic Notation Chemniverse From chemniverse.com
Atomic Structure Relation To Nucleon Numbers Isotopes And Isotopic Notation Chemniverse From chemniverse.com
Lets say an isotope of helium has 2 protons and 2 electrons and 2 neutrons meaning that it would have 4 on the top and a 2 on the bottom. Regarding this how do you find the number of isotopes. Atomic number mass number and isotopes. How many protons and neutrons are there in the nucleus of strontium-90. M B 10013 0199 11009 0801 199 882 1081 Da Relative Isotopic Masses. The number of neutrons is not stated.
So 16 plus 16 is 32.
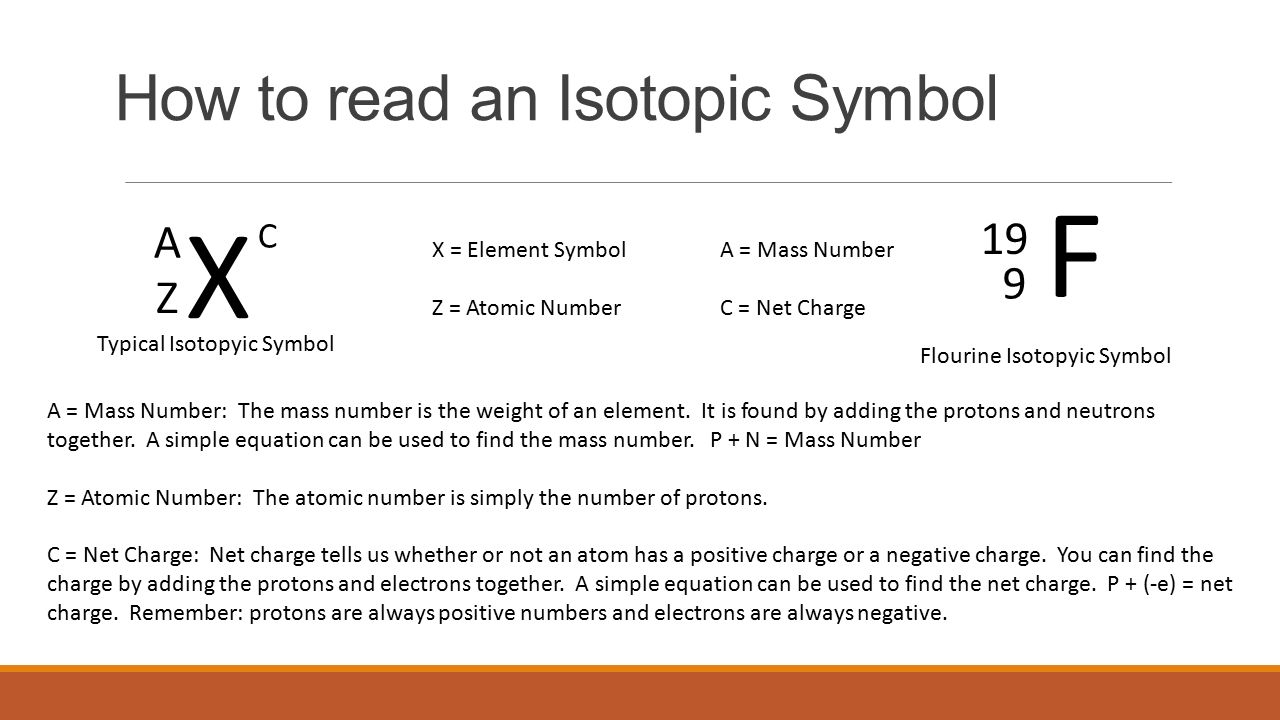
Instead you have to figure it out based on the number of protons or atomic number. Finding Protons and Neutrons in an Isotope Problem. Isotope Notation List the mass number of an element after its name or element symbol. The isotopic notation given is in the form of. Click to see full answer. A N Z Calculations.
 Source: youtube.com
Source: youtube.com
A N Z Calculations. How many protons and neutrons are there in the nucleus of strontium-90. Likewise how do you read isotope names. A N Z. You already know how to find the mass number of an isotope by adding the protons and neutrons.
 Source: youtube.com
Source: youtube.com
The isotopic notation given is in the form of. Regarding this how do you find the number of isotopes. So 16 plus 16 is 32. Where X is the symbol for the element Z is the atomic number number of protons and A is the atomic mass number number of protons plus number of neutrons. Solution The nuclear symbol indicates the composition of the nucleus.
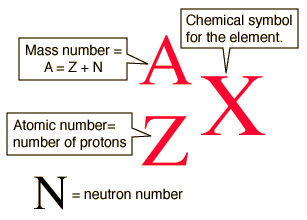 Source: socratic.org
Source: socratic.org
Subtract the atomic number the number of protons from the rounded atomic weight. Learn this topic by watching Isotopes Concept Videos. An isotope is an atom with a different number of neutrons but the same number of protons and electrons. Solution The nuclear symbol indicates the composition of the nucleus. This is the currently selected item.
 Source: slideplayer.com
Source: slideplayer.com
This is the currently selected item. Since sodium Na has an atomic number of 11 this isotope contains. The argon isotope with 22 neutrons. For example the mass and abundance of isotopes of Boron are given below. Learn this topic by watching Isotopes Concept Videos.
 Source: academytalk.com
Source: academytalk.com
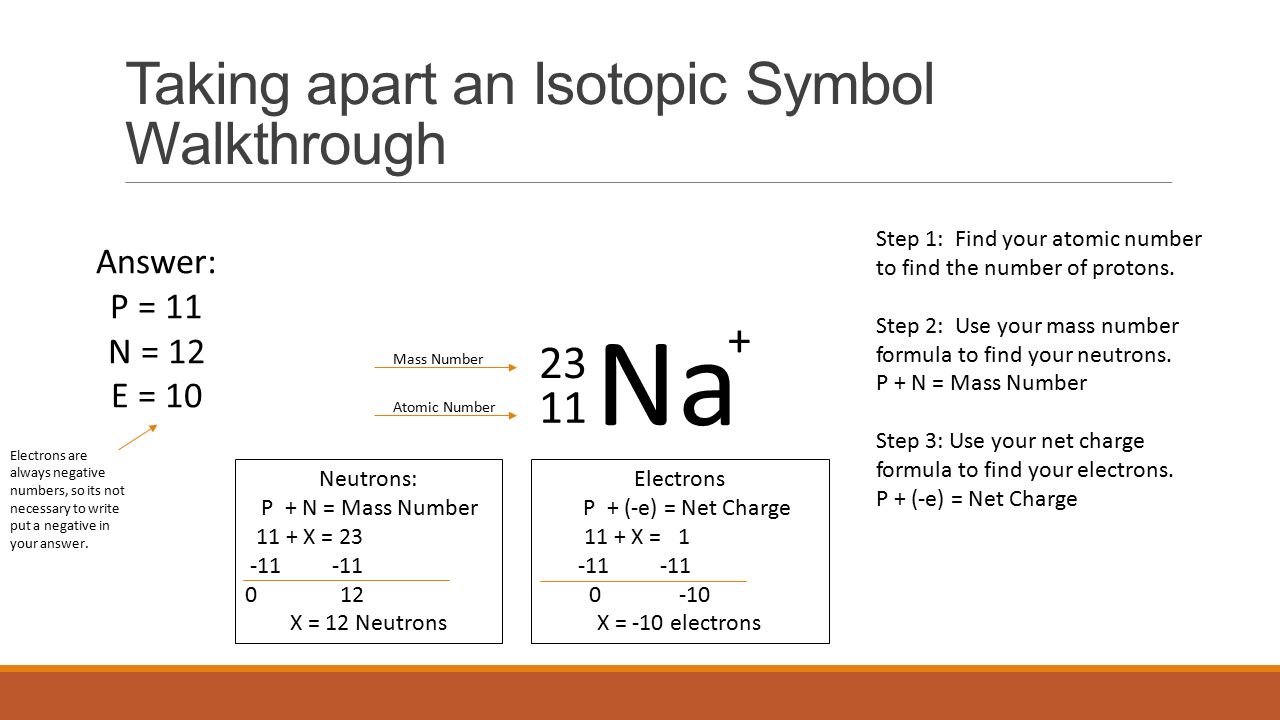
Since sodium Na has an atomic number of 11 this isotope contains. You already know how to find the mass number of an isotope by adding the protons and neutrons. The nuclear symbol of an isotope indicates the number of protons and neutrons in an atom of the element. In this video we are going to go through how to write in isotopic symbol or also known as isotope notation or nuclear notation. The iodine isotope with 74 neutrons.
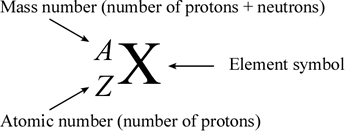 Source: guweb2.gonzaga.edu
Source: guweb2.gonzaga.edu
It does not indicate the number of electrons. One of the harmful species from nuclear fallout is the radioactive isotope of strontium 90 38 Sr assume the super and subscripts line up. Also asked what is an isotopic symbol. Remember an isotope all sulfur atoms are going to have 16 protons but they might have different numbers of neutrons. Isotope notation also known as nuclear notation is important because it allows us to use a visual symbol to easily determine an isotopes mass number atomic number and to determine the number of neutrons and protons in the nucleus.
 Source: youtube.com
Source: youtube.com
When using this notation the symbol of the element must be used to find its atomic number. We can state the relationship between protons neutrons and mass number with the following equation. This isotope is represented using the first symbolism shown in Figure PageIndex2. Solution The nuclear symbol indicates the composition of the nucleus. It does not indicate the number of electrons.
 Source: youtube.com
Source: youtube.com
Instead you have to figure it out based on the number of protons or atomic number. How many protons and neutrons are there in the nucleus of strontium-90. 42 Votes To write the symbol for an isotope place the atomic number as a subscript and the mass number protons plus neutrons as a superscript to the left of the atomic symbol. This gives you the number of neutrons in the most common isotope. To write the symbol for an isotope place the atomic number as a subscript and the mass number protons plus neutrons as a superscript to the left of the atomic symbol.
 Source: slideplayer.com
Source: slideplayer.com
How many protons and neutrons are there in the nucleus of strontium-90. It does not indicate the number of electrons. The phosphorus isotope with 16 neutrons. Finding Protons and Neutrons in an Isotope Problem. The symbols for the two naturally occurring isotopes of chlorine are written as.
 Source: quizlet.com
Source: quizlet.com
You already know how to find the mass number of an isotope by adding the protons and neutrons. How many protons and neutrons are there in the nucleus of strontium-90. To determine the most abundant isotopic form of an element compare given isotopes to the weighted average on the periodic table. What is its isotope notation. To write the symbol for an isotope place the atomic number as a subscript and the mass number protons plus neutrons as a superscript to the left of the atomic symbol.
 Source: youtube.com
Source: youtube.com
A N Z. A N Z Calculations. Regarding this how do you find the number of isotopes. To write the symbol for an isotope place the atomic number as a subscript and the mass number protons plus neutrons as a superscript to the left of the atomic symbol. So this case we have 16 protons and we have 16 neutrons so if you add the protons plus the neutrons together youre going to get your mass number.
 Source: isotopes.gov
Source: isotopes.gov
An isotope is an atom with a different number of neutrons but the same number of protons and electrons. Subscripts and superscripts can be added to an elements symbol to specify a particular isotope of the element and provide other important information. Click to see full answer. So the sulfurs that have different number of neutrons those would be different isotopes. We can state the relationship between protons neutrons and mass number with the following equation.
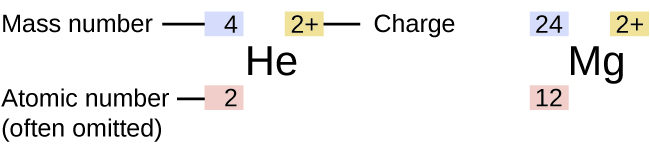 Source: opentextbc.ca
Source: opentextbc.ca
The symbols for the two naturally occurring isotopes of chlorine are written as follows. What is its isotope notation. Each element has a standard number of neutrons that can be found by looking at a periodic table. Since sodium Na has an atomic number of 11 this isotope contains. For example the mass and abundance of isotopes of Boron are given below.
 Source: slideplayer.com
Source: slideplayer.com
Each element has a standard number of neutrons that can be found by looking at a periodic table. Click to see full answer. Where X is the symbol for the element Z is the atomic number number of protons and A is the atomic mass number number of protons plus number of neutrons. Since sodium Na has an atomic number of 11 this isotope contains. Subtract the atomic number the number of protons from the rounded atomic weight.
 Source: slideplayer.com
Source: slideplayer.com
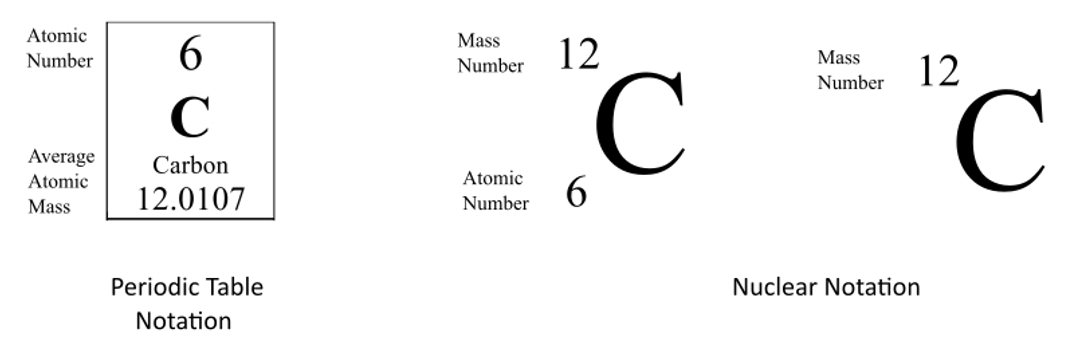
Note the mass number of two isotopes may be the same even though they are different elements. For example the three hydrogen isotopes shown above are H-1 H-2 and H-3. So this case we have 16 protons and we have 16 neutrons so if you add the protons plus the neutrons together youre going to get your mass number. From the periodic table you will get the atomic number on the top left corner of the box. For example the mass and abundance of isotopes of Boron are given below.
 Source: slideplayer.com
Source: slideplayer.com
To write the symbol for an isotope place the atomic number as a subscript and the mass number protons plus neutrons as a superscript to the left of the atomic symbol. Also asked what is an isotopic symbol. Click to see full answer. There are three common ways we can represent an element. Intro to Isotopes A N Z Calcs Abundance Notation Naming Isotopes vs Ions Uses Stability More Chem Help Notation and Naming AZE notation In chemistry naming and notation are essential for clear communication.

Remember an isotope all sulfur atoms are going to have 16 protons but they might have different numbers of neutrons. Since sodium Na has an atomic number of 11 this isotope contains. Use the interactive periodic table at The Berkeley Laboratory Isotopes Project to find what other isotopes of that element exist. This isotope is represented using the first symbolism shown in Figure PageIndex2. An isotope with 6 protons and 7 neutrons is carbon-13 or C-16.
 Source: terpconnect.umd.edu
Source: terpconnect.umd.edu
What is its isotope notation. There are three common ways we can represent an element. The symbols for the two naturally occurring isotopes of chlorine are written as follows. The atomic number is written as a subscript on the left of the element symbol the mass number is written as a superscript on the left of the element symbol and the ionic charge if any appears as a superscript on the right. The nuclear symbol of an isotope indicates the number of protons and neutrons in an atom of the element.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site beneficial, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how to find isotope symbol by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






