Your Lewis symbol for carbon images are ready. Lewis symbol for carbon are a topic that is being searched for and liked by netizens now. You can Download the Lewis symbol for carbon files here. Download all royalty-free images.
If you’re looking for lewis symbol for carbon pictures information connected with to the lewis symbol for carbon keyword, you have pay a visit to the ideal blog. Our website frequently provides you with hints for seeing the maximum quality video and picture content, please kindly surf and locate more enlightening video content and graphics that fit your interests.
Lewis Symbol For Carbon. Lewis Diagrams for Molecules. Clear discussion of Carbon valence electrons lewis dot. Ga P As. Because hydrogen only needs two electrons to fill its valence shell it is an exception to the octet rule.
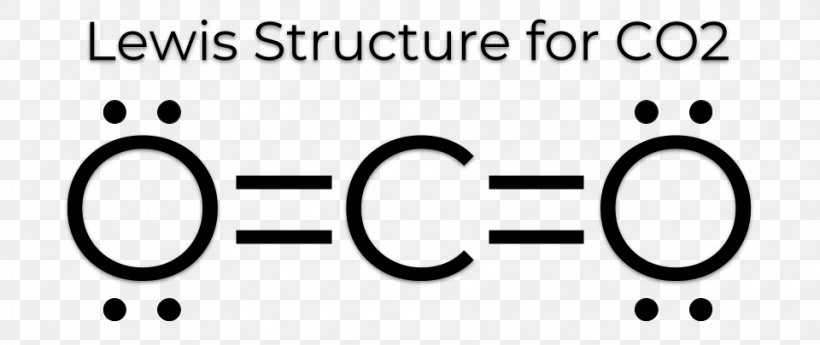 Lewis Structure Carbon Dioxide Resonance Diagram Electron Png 960x404px Lewis Structure Acetylene Area Black And White From favpng.com
Lewis Structure Carbon Dioxide Resonance Diagram Electron Png 960x404px Lewis Structure Acetylene Area Black And White From favpng.com
The Lewis symbol for carbon. 4 4 Section 93 20. The number of bonds which. Draw Lewis structures for atoms ions and simple molecules. Oxygen contains 6 valence electrons which form 2 lone pairs. Arrange the following in terms of increasing electronegativity values.
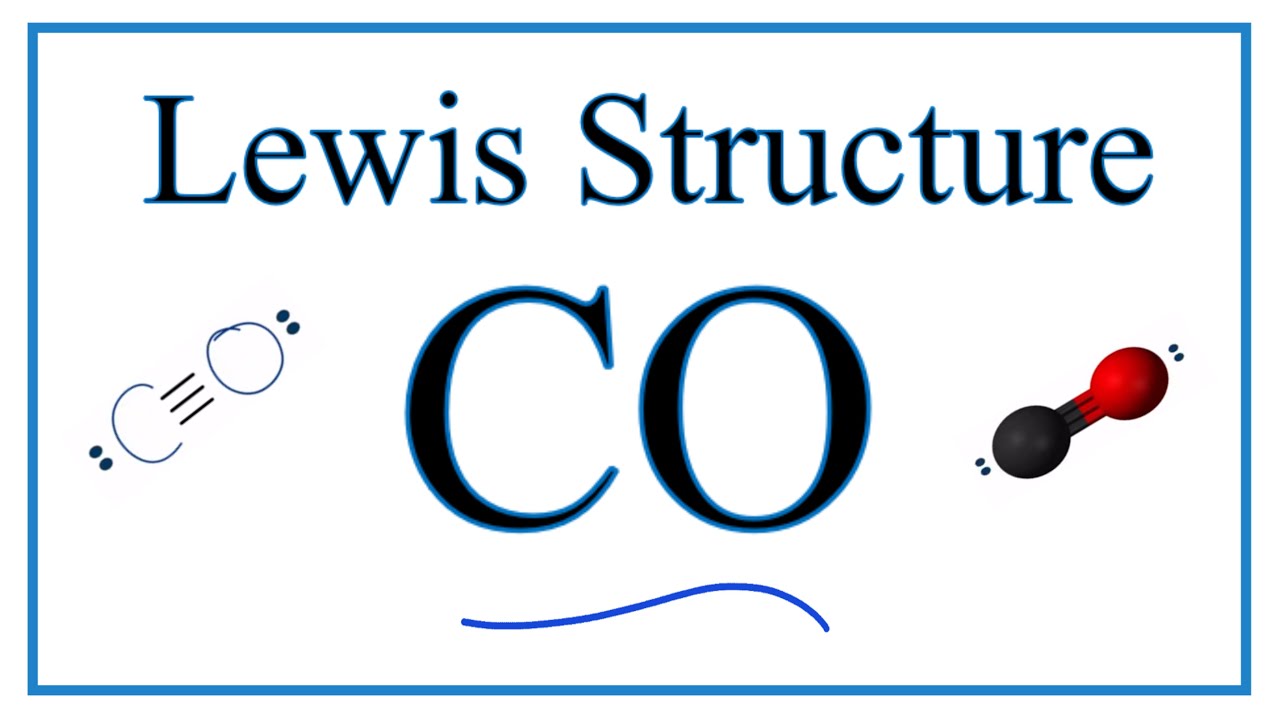
The Lewis structure for CO has 10 valence electrons.
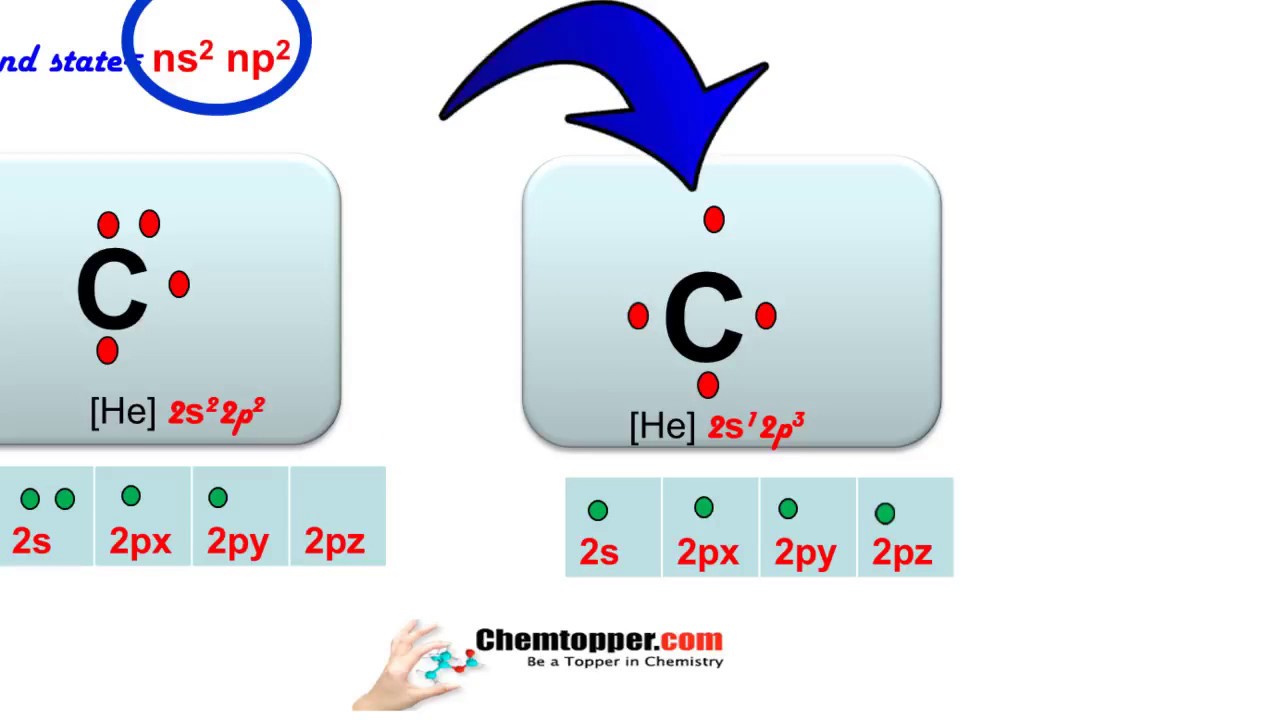
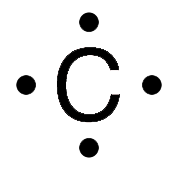
Which statement correctly describes the structure of the whole molecule. Below are Lewis Symbols for various elements. Scientists often create models to represent either a physical or abstract system or structure. Which statement correctly describes the structure of the whole molecule. Lewis Symbols For example the Lewis symbol of carbon depicts a C surrounded by 4 valence electrons because carbon has an electron configuration of 1s22s22p2. Lewis Diagrams for Molecules.
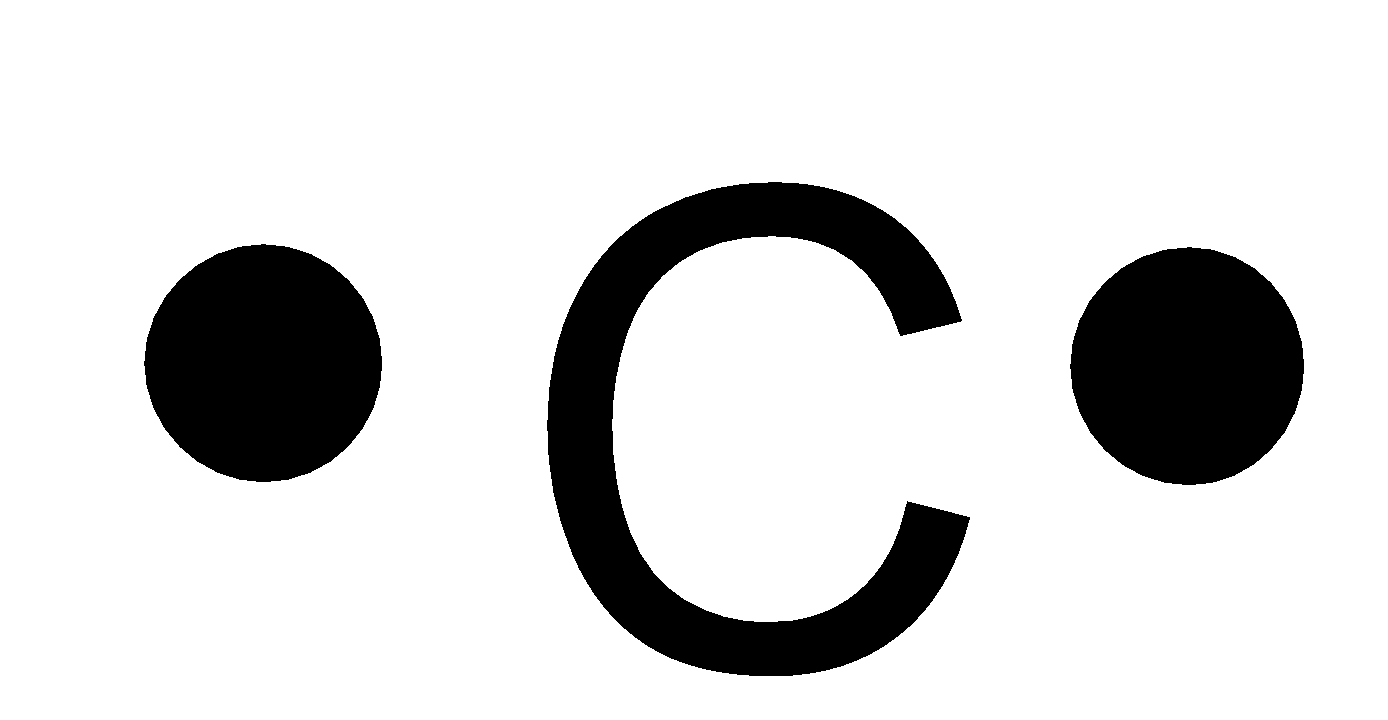 Source: vedantu.com
Source: vedantu.com
Which element will have 5 electrons in its Lewis dot symbol. A clear explanation of valence electrons and how to draw lewis dot symbol of group IV A elements. Lewis Symbols For example the Lewis symbol of carbon depicts a C surrounded by 4 valence electrons because carbon has an electron configuration of 1s22s22p2. Hence oxygen has 6 valence electrons and carbon has 4. Lewis Diagrams for Molecules.
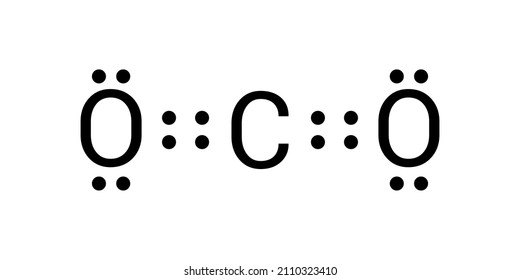 Source: shutterstock.com
Source: shutterstock.com
In C O 2 oxygen belongs to group 16 of the Periodic Table and carbon belongs to group 14 of the Periodic Table. Lewis dot diagram of Carbon This video shows how to use the periodic table to draw Lewis structures and figure out how many valence electrons an atom has. For many common elements the number of dots corresponds to the elements group number. Carbon has four valence electrons and therefore they are drawn on the four sides of a carbon atom as represented in the figures below. What is the Lewis dot diagram for carbon.
 Source: favpng.com
Source: favpng.com
The Lewis symbol for carbon. Ga P As. Carbon is the least electronegative that means it stays at the center. Read complete answer here. These four electrons can be gained by forming four covalent bonds as illustrated here for carbon in CCl 4 carbon tetrachloride and silicon in SiH 4 silane.
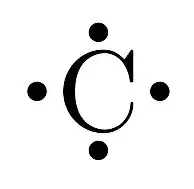 Source: quora.com
Source: quora.com
Which statement correctly describes the structure of the whole molecule. Below are Lewis Symbols for various elements. When we draw the Lewis Structure for the Carbon youll put four dots or valance electrons around the element symbol C. A clear explanation of valence electrons and how to draw lewis dot symbol of group IV A elements. The Lewis structure for oxygen molecule is as shown below- Lewis Structure of Carbon Dioxide C O 2.
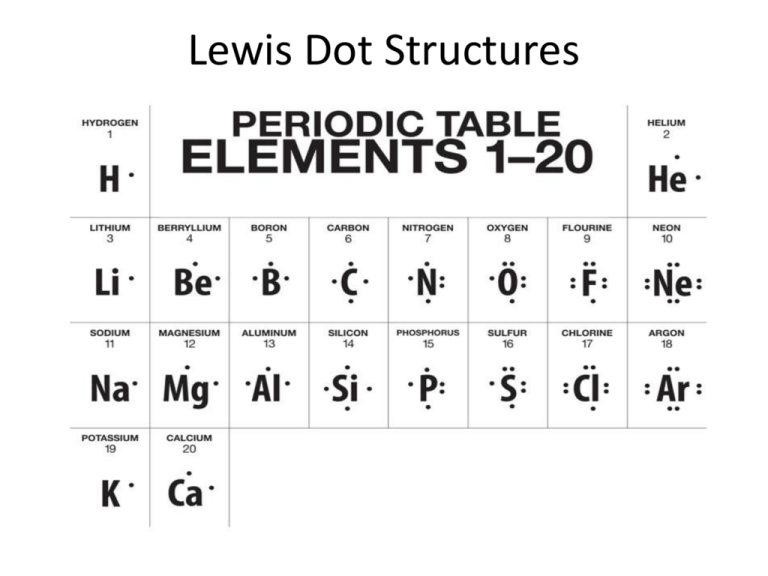 Source: studylib.net
Source: studylib.net
The Lewis symbol for carbon. Carbon has four valence electrons and therefore they are drawn on the four sides of a carbon atom as represented in the figures below. Each of the four valence electrons is represented as a dot. Lewis Structure of CO2 The central atom of this molecule is carbon. How to Draw the Lewis Dot Structure for Carbon monoxide It is helpful if you.
![]() Source: study.com
Source: study.com
The Lewis symbol for the carbon atom shows __ valence electrons. Opo Advertisement Answer 45 5 4 hannahboo Lewis Symbol For Carbon. These four electrons can be gained by forming four covalent bonds as illustrated here for carbon in CCl 4 carbon tetrachloride and silicon in SiH 4 silane. Scientists often create models to represent either a physical or abstract system or structure. Lewis dot diagram of Carbon This video shows how to use the periodic table to draw Lewis structures and figure out how many valence electrons an atom has.
 Source: youtube.com
Source: youtube.com
Because hydrogen only needs two electrons to fill its valence shell it is an exception to the octet rule and only needs to. Ga P As. Hence oxygen has 6 valence electrons and carbon has 4. These four electrons can be gained by forming four covalent bonds as illustrated here for carbon in CCl 4 carbon tetrachloride and silicon in SiH 4 silane. Use Lewis structures as a guide to construct three-dimensional models of small molecules.

CO2 Lewis structure So CO2 4 6 2 16. Which bond is the most polar. The Lewis symbol for carbon. Carbon has four valence electrons and therefore they are drawn on the four sides of a carbon atom as represented in the figures below. What is the maximum number of dots in a Lewis dot structure.
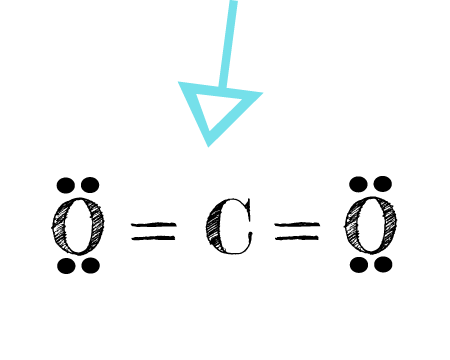 Source: learnwithdrscott.com
Source: learnwithdrscott.com
The electron dot diagram of an element or a molecule is called Lewis structure. CO2 Lewis structure So CO2 4 6 2 16. Ga P As. The Lewis symbol for the carbon atom shows __ valence electrons. The Lewis symbol for carbon.
 Source: youaskweanswer.net
Source: youaskweanswer.net
These four electrons can be gained by forming four covalent bonds as illustrated here for carbon in CCl 4 carbon tetrachloride and silicon in SiH 4 silane. Each of the four valence electrons is represented as a dot. The electron dot diagram of an element or a molecule is called Lewis structure. Hence oxygen has 6 valence electrons and carbon has 4. Lewis dot diagram of Carbon This video shows how to use the periodic table to draw Lewis structures and figure out how many valence electrons an atom has.
 Source: pinterest.com
Source: pinterest.com
Because hydrogen only needs two electrons to fill its valence shell it is an exception to the octet rule and only needs to. It features the distribution of valence electrons around elements. Read complete answer here. The electron dot diagram of an element or a molecule is called Lewis structure. Determine the electron and molecular geometry of the produced molecules.
 Source: youtube.com
Source: youtube.com
So total valence electrons are 16. Also since nitrogen must gain three electrons to achieve a noble-gas configuration it will usually form three covalent bonds. When we draw the Lewis Structure for the Carbon youll put four dots or valance electrons around the element symbol C. Notice the correspondence to each elements group number. Each of the four valence electrons is represented as a dot.
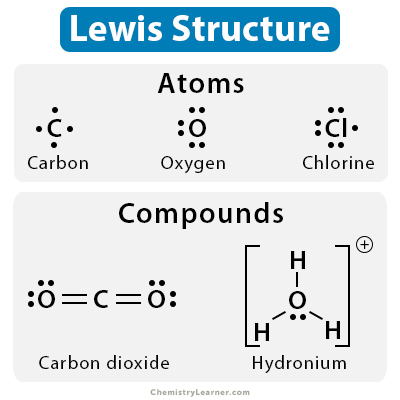 Source: chemistrylearner.com
Source: chemistrylearner.com
For many common elements the number of dots corresponds to the elements group number. Lewis Symbols For example the Lewis symbol of carbon depicts a C surrounded by 4 valence electrons because carbon has an electron configuration of 1s22s22p2. It features the distribution of valence electrons around elements. Read complete answer here. Lewis dot diagram of Carbon This video shows how to use the periodic table to draw Lewis structures and figure out how many valence electrons an atom has.

Opo Advertisement Answer 45 5 4 hannahboo Lewis Symbol For Carbon. It features the distribution of valence electrons around elements. Write the correct Lewis dot structure for O2. For many common elements the number of dots corresponds to the elements group number. Read complete answer here.
 Source: youtube.com
Source: youtube.com
Determine the electron and molecular geometry of the produced molecules. The Lewis structure for oxygen molecule is as shown below- Lewis Structure of Carbon Dioxide C O 2. Also question is what is the Lewis structure for carbon dioxide. A Lewis Symbol is constructed by placing dots representing electrons in the outer energy around the symbol for the element. These four electrons can be gained by forming four covalent bonds as illustrated here for carbon in CCl 4 carbon tetrachloride and silicon in SiH 4 silane.
 Source: youtube.com
Source: youtube.com
Oxygen contains 6 valence electrons which form 2 lone pairs. The maximum number of valence electron dots in the Lewis electron dot diagram is begin align8end align. These four electrons can be gained by forming four covalent bonds as illustrated here for carbon in CCl 4 carbon tetrachloride and silicon in SiH 4 silane. The Lewis structure for oxygen molecule is as shown below- Lewis Structure of Carbon Dioxide C O 2. The Lewis symbol for the carbon atom has __ valence electrons.
 Source: pngwing.com
Source: pngwing.com
Which statement correctly describes the structure of the whole molecule. Notice the correspondence to each elements group number. Thus the Lewis Carbon structure is letter C symbol for Carbon representing the Carbon core of six protons and six neutrons and two s electrons. Each of the four valence electrons is represented as a dot. The Lewis symbol for carbon.
 Source: socratic.org
Source: socratic.org
The Lewis symbol for the nitrogen atom shows __ valence electrons. In C O 2 oxygen belongs to group 16 of the Periodic Table and carbon belongs to group 14 of the Periodic Table. In the structure of Lewis the entire atom with the exception of the valence electrons is represented by the symbol of the element and the valence electrons are represented by points. Dig into the news of lewis symbol for carbon. Lewis Structure of CO2 The central atom of this molecule is carbon.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site convienient, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title lewis symbol for carbon by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






