Your Lewis symbol for n images are ready. Lewis symbol for n are a topic that is being searched for and liked by netizens today. You can Find and Download the Lewis symbol for n files here. Find and Download all royalty-free photos.
If you’re looking for lewis symbol for n pictures information related to the lewis symbol for n interest, you have come to the ideal blog. Our website always gives you suggestions for seeking the highest quality video and picture content, please kindly search and locate more enlightening video articles and images that match your interests.
Lewis Symbol For N. GN Lewis introduced symbols to represent the valence electrons in the atomsThey are called Lewis symbols where valence electrons are shown as dots surrounding the symbol of atom or ion. Figure PageIndex1 shows the Lewis symbols for the elements of the third period of the periodic table. On December 31 2010 Gonzalez. Once again only valence electrons are shown.

For the N3- Lewis structure calculate the total number of valence electrons for the N3- molecule. Mg belongs to 3rd period 2nd group of periodic table has 2 electrons in the valence shell therefore lewis structure is. When you draw the Lewis structure for Nitrogen youll put five dots or valance electrons around the element symbol N. Lewis Diagrams for Molecules. 98 452 ratings Sign up for free to keep watching this solution Sign up for free. Write an electron configuration for N Then write a Lewis symbol for N and show which electrons from the electron configuration are included in the Lewis symbol.
Then write the Lewis symbol for N and show which electrons from the electron configuration are included in the Lewis symbol.
Would it be N with 8 valence electrons around and with brackets and the 3- on the top right. Drawing the Lewis Structure for N 3-Video. FREE Expert Solution Show answer Answer. Figure PageIndex1 shows the Lewis symbols for the elements of the third period of the periodic table. For the N3- Lewis structure calculate the total number of valence electrons for the N3- molecule. Oxygen and other atoms in group 16 obtain an octet by forming two covalent bonds.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
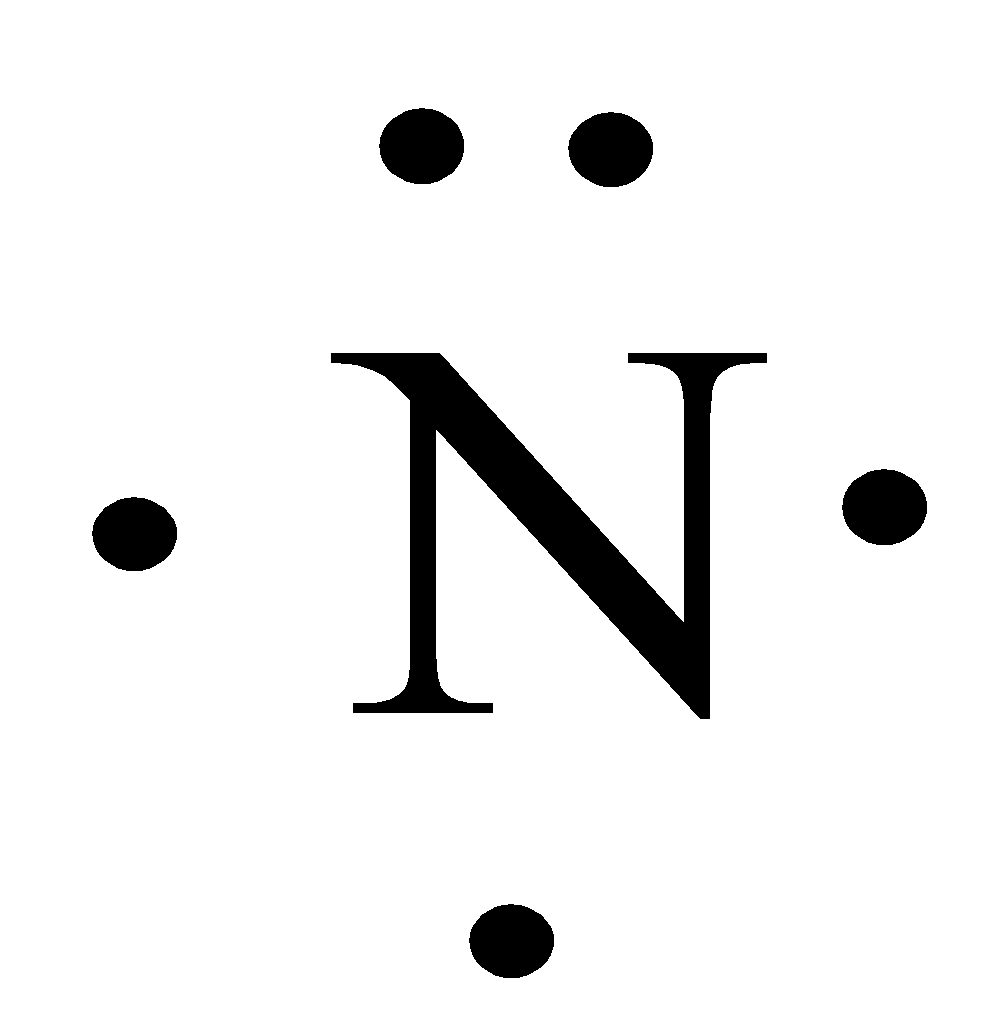
_12Mg 282 Lewis symbol _11Mg 281 Lewis symbol _5B 23 Lewis symbol _8O 26 Lewis symbol _7N 2 5 Lewis symbol _35Br 28187 Lewis symbol. Figure PageIndex1 shows the Lewis symbols for the elements of the third period of the periodic table. A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons. Answer the electron configuration. Nitrogen atom has 5 valence electrons so its Lewis dot symbol for N is.
 Source: techiescientist.com
Source: techiescientist.com
Oxygen and other atoms in group 16 obtain an octet by forming two covalent bonds. Drawing the Lewis Structure for N 3-Video. For the Be2 structure use the periodic table to find the total number of valence electrons for Be. Figure PageIndex1 shows the Lewis symbols for the elements of the third period of the periodic table. Thus the electron configuration of N is.
 Source: youtube.com
Source: youtube.com
Continue watching with Facebook Continue. Group 15 elements such as nitrogen have five valence electrons in the atomic Lewis symbol. Two a pair of valence electrons that are not used to. Electron dots are typically arranged in four pairs. And two on the top and bottom also.
 Source: youtube.com
Source: youtube.com
Drawing the Lewis Structure for N 3-Video. Once we know how many valence electrons there are in Bery. A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons. The following algorithm can be used to construct Lewis diagrams of most molecules. 98 452 ratings Sign up for free to keep watching this solution Sign up for free.
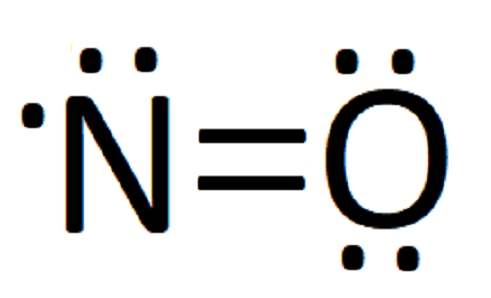 Source: sciencetrends.com
Source: sciencetrends.com
And two on the top and bottom also. We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. Group 15 elements such as nitrogen have five valence electrons in the atomic Lewis symbol. When you draw the Lewis structure for Nitrogen youll put five dots or valance electrons around the element symbol N. Lines denote bonded electron pairs whereas dots are reserved for unbounded electrons.
 Source: youtube.com
Source: youtube.com
Helpful Not Helpful. Two a pair of valence electrons that are not used to. Click to see full answer. Symbol for an element or monatomic ion that uses a dot to represent each valence electron in the element or ion lone pair. Molecules can be depicted by Lewis Diagrams by placing dots or lines around the constituent elemental symbols.
 Source: learnwithdrscott.com
Source: learnwithdrscott.com
Study Hab is a community of 2557630 amazing learners Were a place where learners ask for help for their tasks and share their knowledge. Das Symbol N leitet sich von der lateinischen Bezeichnung nitrogenium ab von altgriechisch νίτρον nítron Laugensalz und -gen meist als Salpeterbildner übersetzt. Lewis Diagrams for Molecules. This video shows how to use the periodic table to draw Lewis structures and figure out how many valence electrons an atom has. FREE Expert Solution Show answer Answer.
 Source: youtube.com
Source: youtube.com
The number of lewis dots to put around the elements symbol is equal to its valence electrons and its valence electron can be determined by its group number in the. Nitrogen atom has 5 valence electrons so its Lewis dot symbol for N is. Since it is in Group 5 it will have 5 valence electrons. Molecules can be depicted by Lewis Diagrams by placing dots or lines around the constituent elemental symbols. Write an electron configuration for N Then write a Lewis symbol for N and show which electrons from the electron configuration are included in the Lewis symbol.
 Source: socratic.org
Source: socratic.org
98 452 ratings Sign up for free to keep watching this solution Sign up for free. Lewis Diagrams for Molecules. Helpful Not Helpful. For the Be2 structure use the periodic table to find the total number of valence electrons for Be. Once we know how many valence electrons there are in Bery.
 Source: emedicalprep.com
Source: emedicalprep.com
Drawing the Lewis Structure for N 3-Video. For the N3- Lewis structure calculate the total number of valence electrons for the N3- molecule. Since it is in Group 5 it will have 5 valence electrons. To obtain an octet these atoms form three covalent bonds as in NH 3 ammonia. Would it be N with 8 valence electrons around and with brackets and the 3- on the top right.

The following algorithm can be used to construct Lewis diagrams of most molecules. A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons. Thus the electron configuration of N is. The electron configuration of Nitrogen having a symbol N can be known by its atomic number which is 7. 18102017 Lewis-Formeln und ihre Kurzschreibweise Regeln dazu.
 Source: learnwithdrscott.com
Source: learnwithdrscott.com
The sum of products divided by the sum of frequencies equals the weighted means. Oxygen and other atoms in group 16 obtain an octet by forming two covalent bonds. For the Be2 structure use the periodic table to find the total number of valence electrons for Be. To obtain an octet these atoms form three covalent bonds as in NH 3 ammonia. Thus the electron configuration of N is.
 Source: sciencetrends.com
Source: sciencetrends.com
Then write the Lewis symbol for N and show which electrons from the electron configuration are included in the Lewis symbol. Next Post Next The Lewis symbol for Mg is. When you draw the Lewis structure for Nitrogen youll put five dots or valance electrons around the element symbol N. Log in Create account Study Hab. Since it is in Group 5 it will have 5 valence electrons.
 Source: vedantu.com
Source: vedantu.com
We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. The following algorithm can be used to construct Lewis diagrams of most molecules. The number of lewis dots to put around the elements symbol is equal to its valence electrons and its valence electron can be determined by its group number in the. Drawing the Lewis Structure for N 3-In the Lewis Structure for N3- youll need to place a double bonds between the Nitrogen atoms to achieve full outer shells on all atoms while only using the valence electrons available for the molecule. On January 1 2011 Reese Company granted Jack Buchanan an employee an option to buy 100 shares of Reese Coshares for 40 per share the option exercisable for 5 years.

Answer the electron configuration. Drawing the Lewis Structure for N 3-In the Lewis Structure for N3- youll need to place a double bonds between the Nitrogen atoms to achieve full outer shells on all atoms while only using the valence electrons available for the molecule. A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons. One lone pair and three unpaired electrons. 18102017 Lewis-Formeln und ihre Kurzschreibweise Regeln dazu.
 Source: quizlet.com
Source: quizlet.com
When you draw the Lewis structure for Nitrogen youll put five dots or valance electrons around the element symbol N. Oxygen and other atoms in group 16 obtain an octet by forming two covalent bonds. Symbol for an element or monatomic ion that uses a dot to represent each valence electron in the element or ion lone pair. Next Post Next The Lewis symbol for Mg is. Lines denote bonded electron pairs whereas dots are reserved for unbounded electrons.

Thus the electron configuration of N is. Lines denote bonded electron pairs whereas dots are reserved for unbounded electrons. We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. For the N3- Lewis structure calculate the total number of valence electrons for the N3- molecule. Study Hab is a community of 2557630 amazing learners Were a place where learners ask for help for their tasks and share their knowledge.
 Source: youtube.com
Source: youtube.com
And two on the top and bottom also. Lines denote bonded electron pairs whereas dots are reserved for unbounded electrons. We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. Mg belongs to 3rd period 2nd group of periodic table has 2 electrons in the valence shell therefore lewis structure is. Two a pair of valence electrons that are not used to.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site adventageous, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title lewis symbol for n by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.





