Your Lewis symbol for nitrogen images are available in this site. Lewis symbol for nitrogen are a topic that is being searched for and liked by netizens today. You can Get the Lewis symbol for nitrogen files here. Download all royalty-free vectors.
If you’re searching for lewis symbol for nitrogen images information linked to the lewis symbol for nitrogen interest, you have visit the ideal blog. Our site always gives you suggestions for seeing the maximum quality video and picture content, please kindly hunt and locate more informative video content and images that fit your interests.
Lewis Symbol For Nitrogen. Die drei Atome des Kohlenstoffdioxids können nun auf zwei unterschiedliche Weisen angeordnet werden. The Lewis dot symbol explains why nitrogen with three unpaired valence electrons tends to form compounds in which it shares the unpaired electrons to form three bonds. The number of bonds which nitrogen usually forms in order to complete its valence shell and obey the octet rule is ___. NCERT NCERT Exemplar.
 No Lewis Dot Structure Science Trends From sciencetrends.com
No Lewis Dot Structure Science Trends From sciencetrends.com
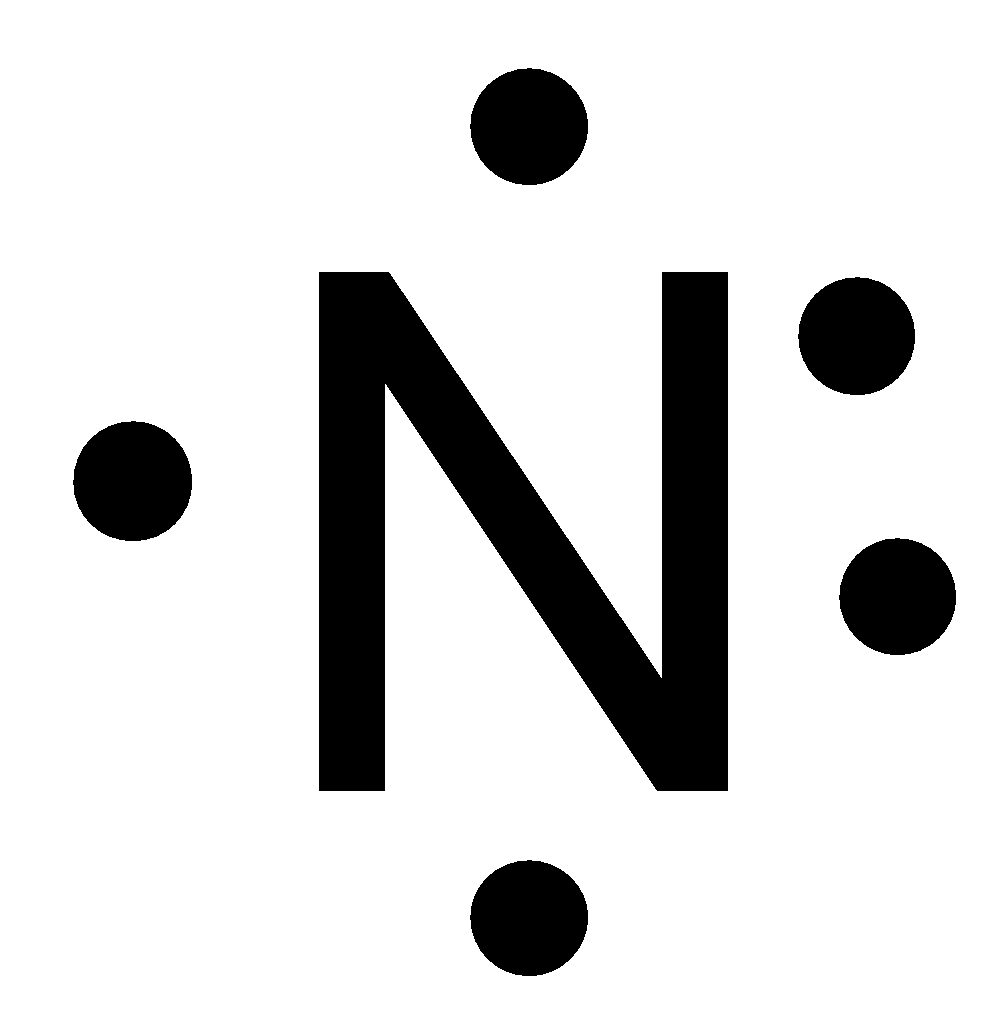
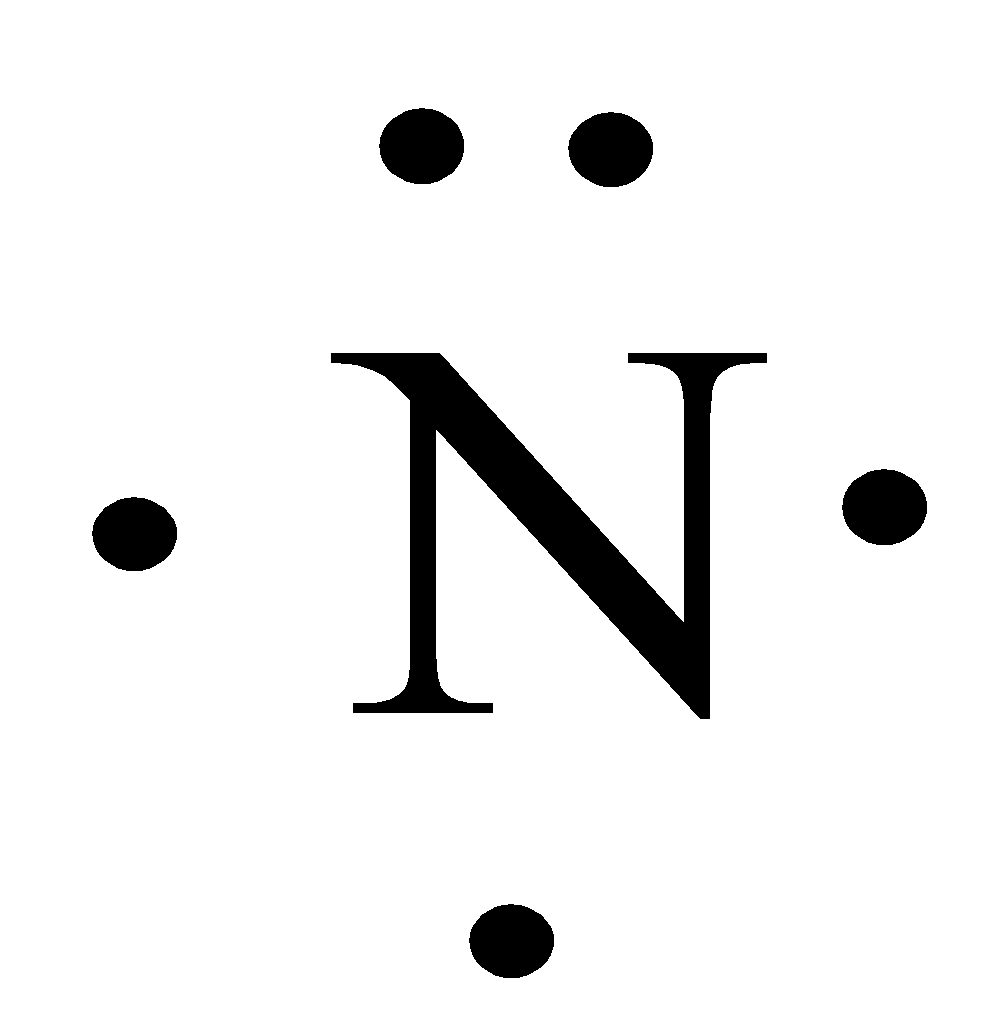
The Lewis symbol for the nitrogen atom shows __ valence electrons. 2 The molecule nitric oxide is naturally present in the human body. C O O O C O 1 2 Die. A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons. Characterize EACH of the three given statements as being TRUE or FALSE and then indicate the collective true-false status of the statements using the choices provided. That means in my Trojan there are three single electrons on one lone pair off electron.
Consider the symbol for nitrogen in Figure PageIndex2.
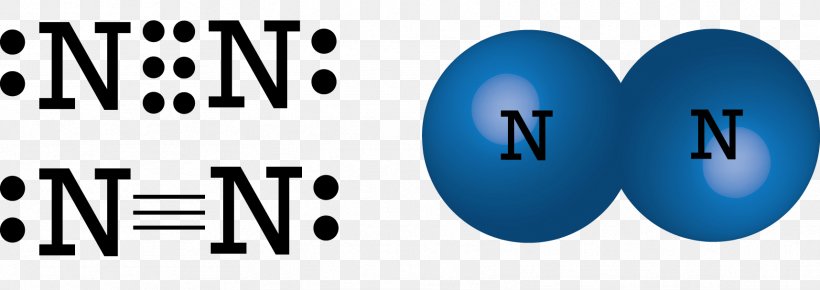
One lone pair and three unpaired electrons. C O O O C O 1 2 Die. 5 4 Section 93 23. Louis symbol is a chemical symbol for him out of which is surrounded by one or more thoughts that we present the number off Valence electrons for night Trojan the Louis symbol will be 1234 Why you and six. Lastly there is a single unpaired electron on the nitrogen atom. Nitric oxide is composed of a single nitrogen atom that is bonded to a nitrogen atom.
 Source: favpng.com
Source: favpng.com
Let us draw the Lewis dot structures of Nitrogen Monoxide NO. Click to see full answer Considering this what are electron dot diagrams used for. Drawing the Lewis Structure for N 3-In the Lewis Structure for N3- youll need to place a double bonds between the Nitrogen atoms to achieve full outer shells on all atoms while only using the valence electrons available for the molecule. The number of bonds which nitrogen usually forms in order to complete its valence shell and obey the octet rule is ___. Die Summe aller Valenzelektronen beträgt im Kohlenstoffdioxid 1 4 2 6 16.
 Source: techiescientist.com
Source: techiescientist.com
The Lewis dot structure of a nitrogen atom would be the capitol letter N with the five valence electrons represented by two dots above it one to the left right and bottom of it. C O O O C O 1 2 Die. Lewis Schreibweise einfach erklärt. Nitric oxide is composed of a single nitrogen atom that is bonded to a nitrogen atom. Click here to get an answer to your question what is the Lewis symbol for nitrogen aayushsh aayushsh 23072017 Chemistry Secondary School answered What is the Lewis symbol for nitrogen 2 See answers.
 Source: youtube.com
Source: youtube.com
NCERT P Bahadur IIT-JEE Previous Year Narendra Awasthi MS Chauhan. Figure 79 shows the Lewis symbols for the elements of the third period of the periodic table. Oh next one We have oxygen. The number of bonds which nitrogen usually forms in order to complete its valence shell and obey the octet rule is ___. To obtain an octet these atoms form three covalent bonds as in NH 3 ammonia.
 Source: docbrown.info
Source: docbrown.info
Draw the lewis symbol for Nitrogen and Sulphur. Drawing the Lewis Structure for N 3-In the Lewis Structure for N3- youll need to place a double bonds between the Nitrogen atoms to achieve full outer shells on all atoms while only using the valence electrons available for the molecule. NCERT NCERT Exemplar. Click here to get an answer to your question what is the Lewis symbol for nitrogen aayushsh aayushsh 23072017 Chemistry Secondary School answered What is the Lewis symbol for nitrogen 2 See answers. One lone pair and three unpaired electrons.
 Source: youtube.com
Source: youtube.com
Characterize EACH of the three given statements as being TRUE or FALSE and then indicate the collective true-false status of the statements using the choices provided. Lewis structure for NO would look like. Nitric oxide is composed of a single nitrogen atom that is bonded to a nitrogen atom. One lone pair and three unpaired electrons. 5 4 Section 93 23.
 Source: brainly.com
Source: brainly.com
Drawing the Lewis Structure for N 3-In the Lewis Structure for N3- youll need to place a double bonds between the Nitrogen atoms to achieve full outer shells on all atoms while only using the valence electrons available for the molecule. Group 15 elements such as nitrogen have five valence electrons in the atomic Lewis symbol. 0011 In der Chemie wird die Lewis Formel auch Elektronenformel genannt. Die drei Atome des Kohlenstoffdioxids können nun auf zwei unterschiedliche Weisen angeordnet werden. The Lewis dot symbol explains why nitrogen with three unpaired valence electrons tends to form compounds in which it shares the unpaired electrons to form three bonds.

Zur Stelle im Video springen. Basierend auf der Oktettregel und den unterschiedlichen Bindungsarten in der Chemie werden in der Lewis Schreibweise auch. Draw the lewis symbol for Nitrogen and Sulphur. 5 4 Section 93 23. Zur Stelle im Video springen.
 Source: brainly.com
Source: brainly.com
5 4 Section 93 23. We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. 0011 In der Chemie wird die Lewis Formel auch Elektronenformel genannt. Consider the symbol for nitrogen in Figure PageIndex2. Click to see full answer Considering this what are electron dot diagrams used for.
 Source: sciencetrends.com
Source: sciencetrends.com
A double bar between two chemical symbols means that nitrogen and oxygen share double bond2 pairs of electrons. C O O O C O 1 2 Die. Die Summe aller Valenzelektronen beträgt im Kohlenstoffdioxid 1 4 2 6 16. Characterize EACH of the three given statements as being TRUE or FALSE and then indicate the collective true-false status of the statements using the choices provided. Lewis Schreibweise einfach erklärt.
 Source: youtube.com
Source: youtube.com
NCERT DC Pandey Sunil Batra HC Verma Pradeep Errorless. Oh next one We have oxygen. The Lewis dot structure of a nitrogen atom would be the capitol letter N with the five valence electrons represented by two dots above it one to the left right and bottom of it. 3 The acronym ORP stands for odor replication. Lewis Schreibweise einfach erklärt.
 Source: youtube.com
Source: youtube.com
C O O O C O 1 2 Die. NCERT DC Pandey Sunil Batra HC Verma Pradeep Errorless. This video shows how to use the periodic table to draw Lewis structures and figure out how many valence electrons an atom has. Consider the symbol for nitrogen in Figure PageIndex2. Louis symbol is a chemical symbol for him out of which is surrounded by one or more thoughts that we present the number off Valence electrons for night Trojan the Louis symbol will be 1234 Why you and six.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
Lastly there is a single unpaired electron on the nitrogen atom. When you draw the Lewis structure for Nitrogen youll put five dots or valance electrons around the element symbol N. Draw the lewis symbol for Nitrogen and Sulphur. 2 The molecule nitric oxide is naturally present in the human body. Drawing the Lewis Structure for N 3-In the Lewis Structure for N3- youll need to place a double bonds between the Nitrogen atoms to achieve full outer shells on all atoms while only using the valence electrons available for the molecule.
 Source: vedantu.com
Source: vedantu.com
NCERT DC Pandey Sunil Batra HC Verma Pradeep Errorless. C O O O C O 1 2 Die. When you draw the Lewis structure for Nitrogen youll put five dots or valance electrons around the element symbol N. Lewis structure for NO would look like. Lewis Schreibweise einfach erklärt.
 Source: vedantu.com
Source: vedantu.com
Figure 79 shows the Lewis symbols for the elements of the third period of the periodic table. Lewis Schreibweise einfach erklärt. This video shows how to use the periodic table to draw Lewis structures and figure out how many valence electrons an atom has. Figure 79 shows the Lewis symbols for the elements of the third period of the periodic table. Calculate the of electrons in π bonds pi bonds multiple bonds using formula 1 in the article entitled Lewis Structures and the Octet Rule.
 Source: pinterest.com
Source: pinterest.com
To obtain an octet these atoms form three covalent bonds as in NH 3 ammonia. The Lewis symbol for the nitrogen atom shows __ valence electrons. Lewis structure for NO would look like. Die Summe aller Valenzelektronen beträgt im Kohlenstoffdioxid 1 4 2 6 16. Group 15 elements such as nitrogen have five valence electrons in the atomic Lewis symbol.
 Source: study.com
Source: study.com
NCERT P Bahadur IIT-JEE Previous Year Narendra Awasthi MS Chauhan. Lewis used the unpaired dots to predict the number of bonds that an element will form in a compound. Oh next one We have oxygen. Complete the Lewis structure for HClO3 from the skeletal template presented below by filling in the. Lastly there is a single unpaired electron on the nitrogen atom.
 Source: socratic.org
Source: socratic.org
Group 15 elements such as nitrogen have five valence electrons in the atomic Lewis symbol. Lewis-Diagramme von Kohlen- und Sauerstoff-Atomen. Calculate the of electrons in π bonds pi bonds multiple bonds using formula 1 in the article entitled Lewis Structures and the Octet Rule. Basierend auf der Oktettregel und den unterschiedlichen Bindungsarten in der Chemie werden in der Lewis Schreibweise auch. After determining how many valence.
 Source: youtube.com
Source: youtube.com
Werden diese 16 Elektronen auf Elektronenpaare verteilt stehen 16. C O O O C O 1 2 Die. 2 The molecule nitric oxide is naturally present in the human body. When you draw the Lewis structure for Nitrogen youll put five dots or valance electrons around the element symbol N. Characterize EACH of the three given statements as being TRUE or FALSE and then indicate the collective true-false status of the statements using the choices provided.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site convienient, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title lewis symbol for nitrogen by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






