Your Lewis symbol for oxygen images are ready. Lewis symbol for oxygen are a topic that is being searched for and liked by netizens now. You can Find and Download the Lewis symbol for oxygen files here. Find and Download all royalty-free photos and vectors.
If you’re searching for lewis symbol for oxygen pictures information linked to the lewis symbol for oxygen interest, you have pay a visit to the right blog. Our site frequently gives you suggestions for seeking the highest quality video and image content, please kindly hunt and locate more informative video articles and graphics that match your interests.
Lewis Symbol For Oxygen. The Lewis electron dot structures of a few molecules are illustrated in this subsection. Lewis Structure Examples. Hence a double bond is formed. The number of electrons in the outer energy level is correlated by simply reading the Group number.
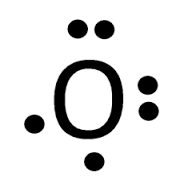 What Kind Of Bond Would The Electron Dot Formula For O 2 Show Socratic From socratic.org
What Kind Of Bond Would The Electron Dot Formula For O 2 Show Socratic From socratic.org
Which of the following is the best Lewis symbol for oxygen. Therefore Lewis Symbols are useful for studying elemental properties and reactions. Hence oxygen has 6 valence electrons. Lewis symbols illustrating the number of valence electrons for each element in the third period of the periodic table. So both the atoms contribute two atoms each for the bond. The number of electrons in the outer energy level is correlated by simply reading the Group number.
Besides how do you write the Lewis symbol.
The central atom of this molecule is carbon. How to Draw the Lewis Dot Structure for O 2- Oxide ion Watch later. A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons. Therefore Lewis Symbols are useful for studying elemental properties and reactions. Learn this topic by watching Lewis Dot Structures. Also what is the meaning of Lewis symbol.
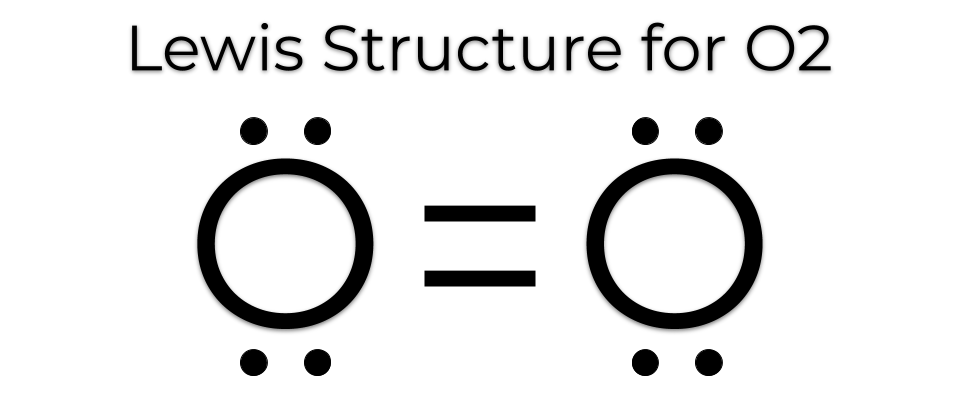 Source: makethebrainhappy.com
Source: makethebrainhappy.com
The oxygen atom is also bonded to a hydrogen atom. Group 16 elements such as oxygen and other atoms obtain an octet by forming two covalent bonds - like bonding with two hydrogen atoms in H 2 O water. Lewis Electron Dot Structure for the molecule. A Lewis symbol is a symbol in which the electrons in the valence shell of an atom or simple ion are represented by dots placed around the letter symbol of the element. Chemistry questions and answers.
 Source: techiescientist.com
Source: techiescientist.com
Previous Answers nitrogen nitrogen moblo-gas covalent e shown other two Correct Correct Part B How many electrons should be shown in the Lewis symbol. A Lewis Symbol consists of the element symbol surrounded by dots to represent the number of electrons in the outer energy level as represented by a Bohr Diagram. Oxygen contains 6 valence electrons which form 2 lone pairs. Besides how do you write the Lewis symbol. Step 1 Calculating the total valence electrons.
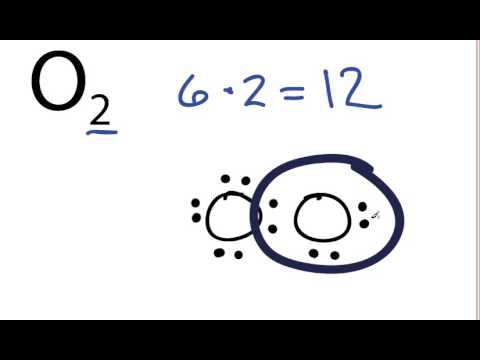 Source: youtube.com
Source: youtube.com
How to Draw the Lewis Dot Structure for O 2- Oxide ion Watch later. Therefore Lewis Symbols are useful for studying elemental properties and reactions. This is followed by a plus sign and the number one point five followed by two oxygen atoms bonded together with a double bond and each with two lone pairs of electrons. So both the atoms contribute two atoms each for the bond. Group 15 elements such as nitrogen have five valence electrons in the atomic Lewis symbol.

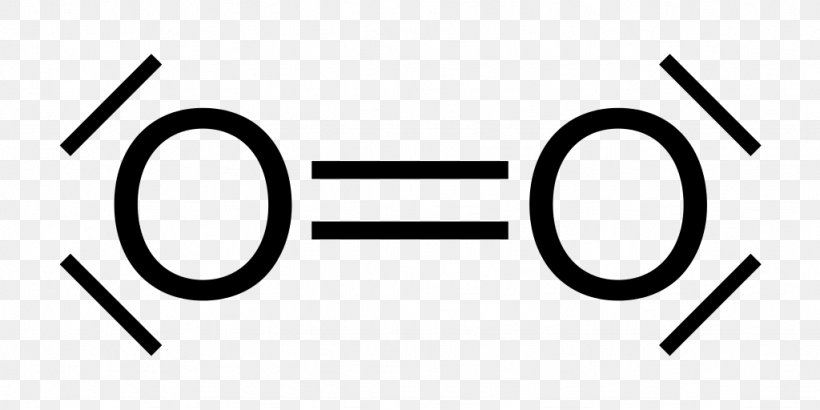
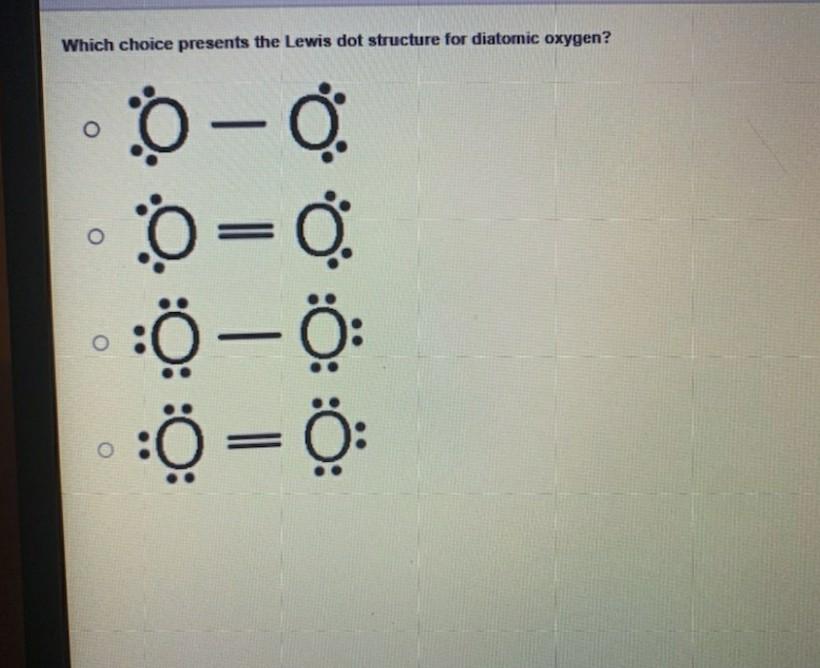
The Lewis electron dot structures of a few molecules are illustrated in this subsection. View Available Hint s ence the le using ogen O ooooo có. Lewis symbols for oxygen fluorine. In O 2 there are only two oxygen atoms. Lewis Electron Dot Structure for O2 molecule An oxygen atom has 6 valence electrons in the valence shell and so it needs 2 more to complete the octet.
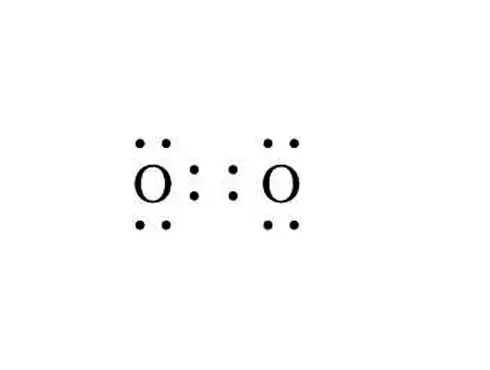 Source: quora.com
Source: quora.com
Lewis structures also known as Lewis dot formulas Lewis dot structures electron dot structures or Lewis electron dot structures LEDS are diagrams that show the bonding between atoms of a molecule as well as the lone pairs of electrons that may exist in the molecule. To obtain an octet these atoms form three covalent bonds as in NH 3 ammonia. The number of electrons in the outer energy level is correlated by simply reading the Group number. Lewis Structure of oxygen O 2 Oxygen belongs to group 16 of the Periodic Table. Therefore Lewis Symbols are useful for studying elemental properties and reactions.
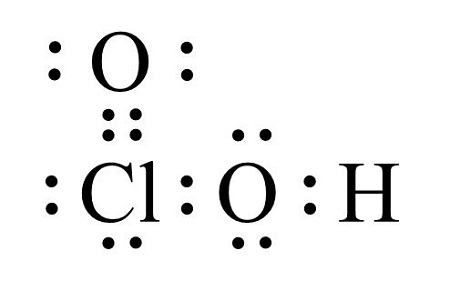 Source: study.com
Source: study.com
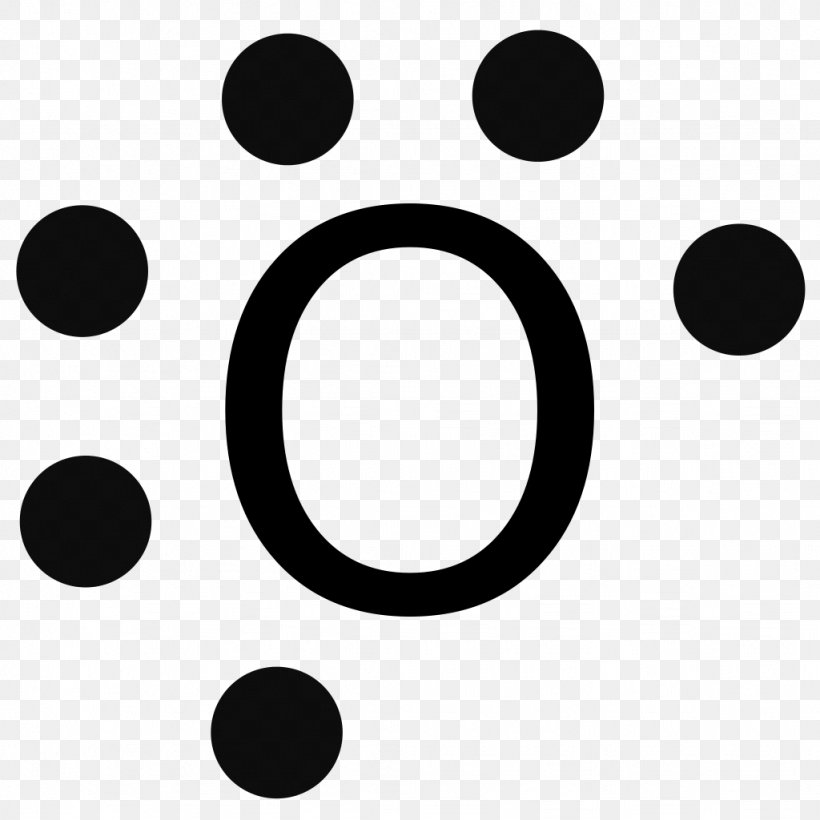
Due to oxygens high electronegativity affinity for electrons the pure element is nearly exclusively found in either this. For example oxygen has 6 valence electrons so we write the symbol O for oxygen and surround it with 6 dots. Previous Answers nitrogen nitrogen moblo-gas covalent e shown other two Correct Correct Part B How many electrons should be shown in the Lewis symbol. Hence oxygen has 6 valence electrons. Since it is bonded to only one carbon atom it must form a double bond.
 Source: favpng.com
Source: favpng.com
Besides how do you write the Lewis symbol. The omitted electrons are those in filled energy levels which do not contribute to the chemical properties of the species in question. One lone pair and three unpaired electrons. Previous Answers nitrogen nitrogen moblo-gas covalent e shown other two Correct Correct Part B How many electrons should be shown in the Lewis symbol. Oxygen and other atoms in group 16 obtain an octet by forming two covalent bonds.
A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons. Due to oxygens high electronegativity affinity for electrons the pure element is nearly exclusively found in either this. Lewis Symbols are simplified Bohr diagrams which only display electrons in the outermost energy level. A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons. In order that the same number of electrons would be donated as accepted we need 2 Al atoms 2 3e donated and 3 O atoms 3 2e accepted.
 Source: favpng.com
Source: favpng.com
So put the Carbon in the middle and then set the oxygen either side of that. Since it is bonded to only one carbon atom it must form a double bond. The central atom of this molecule is carbon. In order that the same number of electrons would be donated as accepted we need 2 Al atoms 2 3e donated and 3 O atoms 3 2e accepted. Group 15 elements such as nitrogen have five valence electrons in the atomic Lewis symbol.
 Source: quora.com
Source: quora.com
So both the atoms contribute two atoms each for the bond. In O 2 total number of valence electrons 6 2 12 valence electrons Step 2 Determination of the central metal atom. Step 1 Calculating the total valence electrons. For example oxygen has 6 valence electrons so we write the symbol O for oxygen and surround it with 6 dots. We first write down Lewis diagrams for each atom involved.

Lewis symbols for oxygen fluorine. We now see that each O atom needs 2 electrons to make up an octet while each Al atom has 3 electrons to donate. Hence a double bond is formed. So put the Carbon in the middle and then set the oxygen either side of that. So according to the lewis dot structure of OF2 oxygen is the central atom and it has 2 bonded pair electrons and 2 lone pairs of electrons.
 Source: learnwithdrscott.com
Source: learnwithdrscott.com
This is followed by a plus sign and the number one point five followed by two oxygen atoms bonded together with a double bond and each with two lone pairs of electrons. Group 15 elements such as nitrogen have five valence electrons in the atomic Lewis symbol. A Lewis symbol is a symbol in which the electrons in the valence shell of an atom or simple ion are represented by dots placed around the letter symbol of the element. The Lewis electron dot structures of a few molecules are illustrated in this subsection. This is followed by a plus sign and the number one point five followed by two oxygen atoms bonded together with a double bond and each with two lone pairs of electrons.
 Source: pinclipart.com
Source: pinclipart.com
We first write down Lewis diagrams for each atom involved. Group 15 elements such as nitrogen have five valence electrons in the atomic Lewis symbol. The oxygen atom is also bonded to a hydrogen atom. Also what is the meaning of Lewis symbol. Lewis symbols can also be used to illustrate the formation of cations from atoms as shown here for sodium and calcium.
 Source: socratic.org
Source: socratic.org
Group 15 elements such as nitrogen have five valence electrons in the atomic Lewis symbol. Step 1 Calculating the total valence electrons. This is followed by a plus sign and the number one point five followed by two oxygen atoms bonded together with a double bond and each with two lone pairs of electrons. Neutral Compounds Concept Videos. The whole process is then.
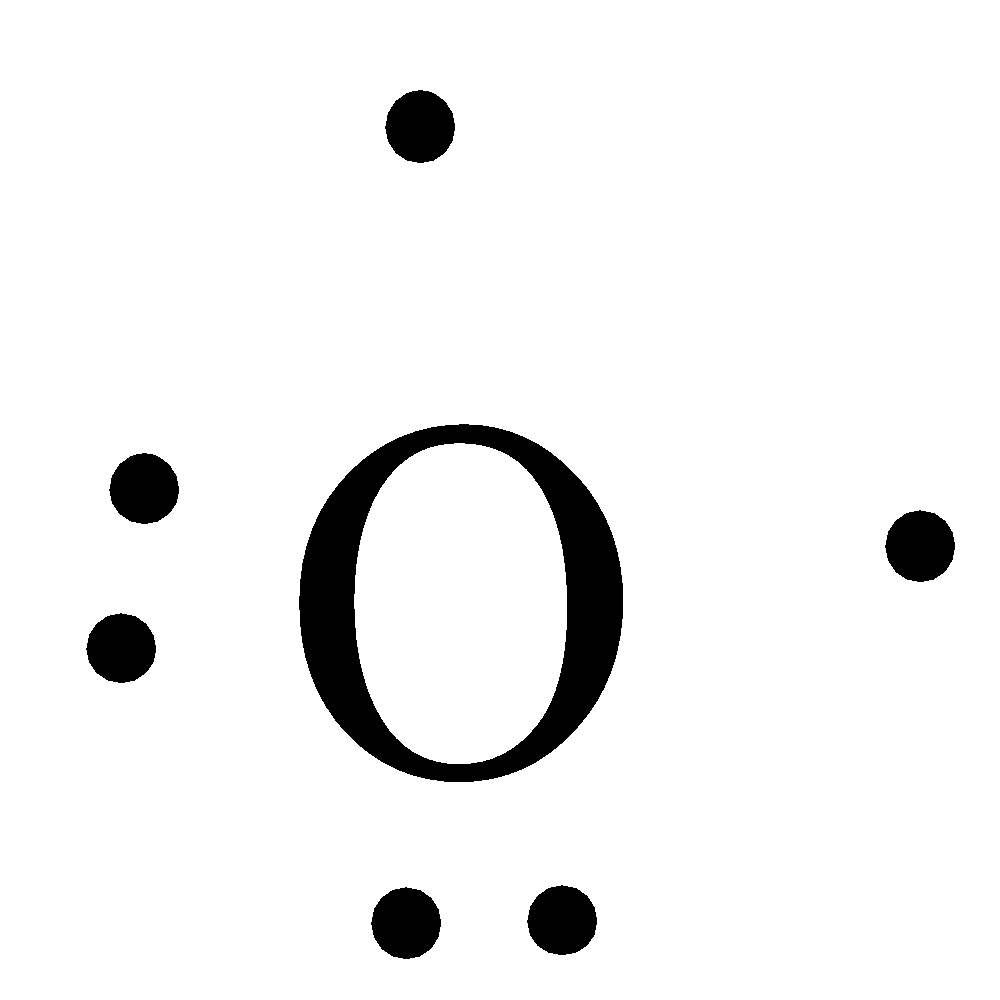 Source: vedantu.com
Source: vedantu.com
The omitted electrons are those in filled energy levels which do not contribute to the chemical properties of the species in question. Lewis Structure Examples. A Lewis Symbol consists of the element symbol surrounded by dots to represent the number of electrons in the outer energy level as represented by a Bohr Diagram. Lewis symbols illustrating the number of valence electrons for each element in the third period of the periodic table. Group 16 elements such as oxygen and other atoms obtain an octet by forming two covalent bonds - like bonding with two hydrogen atoms in H 2 O water.

One lone pair and three unpaired electrons. Lewis Symbols are simplified Bohr diagrams which only display electrons in the outermost energy level. Previous Answers nitrogen nitrogen moblo-gas covalent e shown other two Correct Correct Part B How many electrons should be shown in the Lewis symbol. Lewis Electron Dot Structure for O2 molecule An oxygen atom has 6 valence electrons in the valence shell and so it needs 2 more to complete the octet. In O 2 total number of valence electrons 6 2 12 valence electrons Step 2 Determination of the central metal atom.
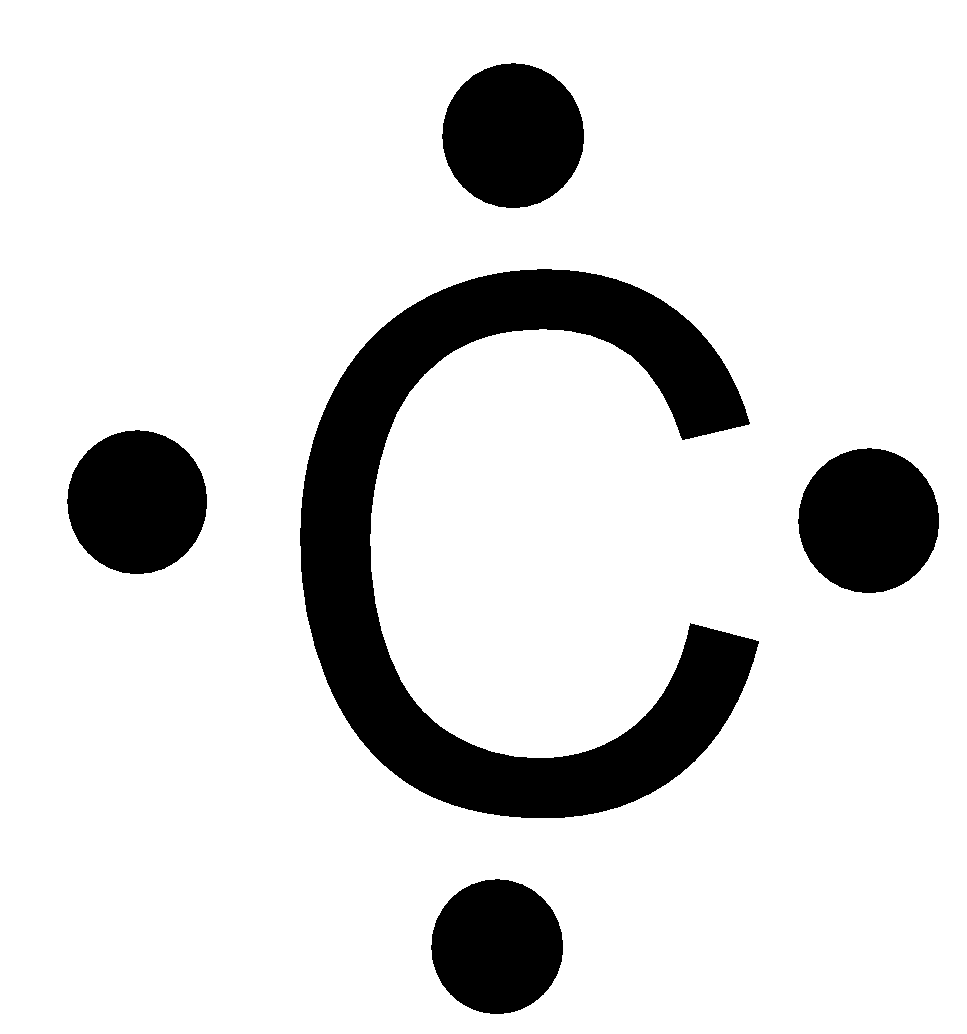 Source: vedantu.com
Source: vedantu.com
In O 2 total number of valence electrons 6 2 12 valence electrons Step 2 Determination of the central metal atom. Step 1 Calculating the total valence electrons. Lewis Symbols are simplified Bohr diagrams which only display electrons in the outermost energy level. We now see that each O atom needs 2 electrons to make up an octet while each Al atom has 3 electrons to donate. So according to the lewis dot structure of OF2 oxygen is the central atom and it has 2 bonded pair electrons and 2 lone pairs of electrons.

Besides how do you write the Lewis symbol. Lewis Symbols are simplified Bohr diagrams which only display electrons in the outermost energy level. Oxygen and other atoms in group 16 obtain an octet by forming two covalent bonds. Neutral Compounds Concept Videos. Lewis symbols can also be used to illustrate the formation of cations from atoms as shown here for sodium and calcium.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site beneficial, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title lewis symbol for oxygen by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






