Your Nitrogen electron dot symbol images are available. Nitrogen electron dot symbol are a topic that is being searched for and liked by netizens now. You can Get the Nitrogen electron dot symbol files here. Find and Download all royalty-free photos and vectors.
If you’re searching for nitrogen electron dot symbol images information linked to the nitrogen electron dot symbol topic, you have pay a visit to the ideal blog. Our site frequently provides you with suggestions for seeing the highest quality video and image content, please kindly search and locate more informative video content and graphics that fit your interests.
Nitrogen Electron Dot Symbol. Lewis dot structures also called electron dot structures are diagrams that describe the chemical bonding between atoms in a molecule. 1 and Example 27. Aluminum - Atomic 13. 2 nitrogen has 5 valence electrons.
 Draw The Lewis Dot Structure For N2 And A Second Structure Showing The Bonds And The Vsepr Shape What Is The Name Of The Shape How Many Lone Pairs Of Electrons Are From study.com
Draw The Lewis Dot Structure For N2 And A Second Structure Showing The Bonds And The Vsepr Shape What Is The Name Of The Shape How Many Lone Pairs Of Electrons Are From study.com
For diatomic nitrogen the Lewis-dot structure correctly predicts that there will be a triple bond between nitrogen atoms. This triple bond is very strong. Remember that nitrogen has five electrons in its outer shell. Aluminum - Atomic 13. Starting from left to. Iodine Selenium Strontium Nitrogen4-2.
Write electron dot symbols for the following atoms.
Instead we need to imagine a square around the chemical symbol. Lets take a closer look at some compounds containing nitrogen. They also display the total number of lone pairs present in each of the atoms that. Electron electrons lefteris valency beryllium. Nitrogen dot electron lewis diagram electrons introductory chemistry which oxygen diagrams symbol dots four. The electronic configuration of Nitrogen is 1 s2 2 s2 2 p3.
 Source: youtube.com
Source: youtube.com
When you draw the Lewis structure for Nitrogen youll put five dots or valance electrons around the element symbol N. A step-by-step explanation of how to draw the Lewis dot structure for N Nitrogen. What is the charge of an ion that contains 25 protons and 27 electrons 4-4. Electron configuration of the atom. By sharing the three 2p electrons nitrogen can form three covalent bonds.
 Source: study.com
Source: study.com
Lewis dot structures also called electron dot structures are diagrams that describe the chemical bonding between atoms in a molecule. The electrons in the valence shell are shown as dots placed around the symbol. Lets take a closer look at some compounds containing nitrogen. Starting from left to. Remember that nitrogen has five electrons in its outer shell.
 Source: quizlet.com
Source: quizlet.com
The electron dot symbol for chlorine is shown at the right. By donating these two electrons. How to draw a dot and cross diagram for ammonia. So total valence electrons 5 12 1 18. Aluminum - Atomic 13.
 Source: techiescientist.com
Source: techiescientist.com
Of dots around the Electron-dot symbol of the atom. Boron which also has three unpaired valence electrons in its Lewis dot symbol also tends to form compounds with three bonds whereas carbon with four unpaired valence electrons in its Lewis dot symbol. The electrons in the valence shell are shown as dots placed around the symbol. The electron dot symbol for chlorine is shown at the right. The representation of molecules in Lewis electron dot structure or just a Lewis structure is in honour of the American chemist Gilbert Newton Lewis.
 Source: sciencetrends.com
Source: sciencetrends.com
Electrons are placed up to two on each side of the elemental symbol for a maximum of eight which is the number of electrons in a filled s and p shell. Write electron dot symbols for the following atoms. The electron dot diagram uses the symbol of the element to replace the nucleus and inner shell electrons. By going through the periodic table we see that the Lewis electron dot diagrams of atoms will never have more than eight dots around the atomic symbol. For diatomic nitrogen the Lewis-dot structure correctly predicts that there will be a triple bond between nitrogen atoms.
 Source: almerja.com
Source: almerja.com
For example we learned that chlorine has 7 valence electrons. Instead we need to imagine a square around the chemical symbol. Iodine Selenium Strontium Nitrogen4-2. For diatomic nitrogen the Lewis-dot structure correctly predicts that there will be a triple bond between nitrogen atoms. The electron dot diagram uses the symbol of the element to replace the nucleus and inner shell electrons.
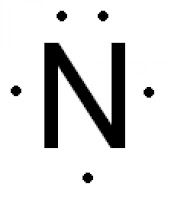 Source: howtodiscuss.com
Source: howtodiscuss.com
NO 2-nitrite ion First count all the valence electrons of one nitrogen and two oxygen atoms and the additional one negative charge for which we will add 1 electron anionic case. Electron configuration of the atom. The representation of molecules in Lewis electron dot structure or just a Lewis structure is in honour of the American chemist Gilbert Newton Lewis. Lets take a closer look at some compounds containing nitrogen. 2 nitrogen has 5 valence electrons.
 Source: toppr.com
Source: toppr.com
The dots should be neatly drawn on the four sides of the. Based on Example 27. Electron Dot Structures - Helpful tools in thinking about bonding. The strength of the triple bond makes the N 2 molecule very stable against chemical change and in fact N 2 is considered to be a chemically inert gas There is a relationship between the number of shared electron pairs and. Try to complete the octet of electrons on all the atoms.
 Source: youtube.com
Source: youtube.com
The electrons in the valence shell are shown as dots placed around the symbol. Nitrogen atom has 5 valence electrons so its Lewis dot symbol for N is. The strength of the triple bond makes the N 2 molecule very stable against chemical change and in fact N 2 is considered to be a chemically inert gas There is a relationship between the number of shared electron pairs and. First draw single bonds between the atoms. The electron dot diagram uses the symbol of the element to replace the nucleus and inner shell electrons.
 Source: socratic.org
Source: socratic.org
By donating these two electrons. 2 nitrogen has 5 valence electrons. For diatomic nitrogen the Lewis-dot structure correctly predicts that there will be a triple bond between nitrogen atoms. Try to complete the octet of electrons on all the atoms. But still the nitrogen atom has a lone pair of electrons form 2s orbital.
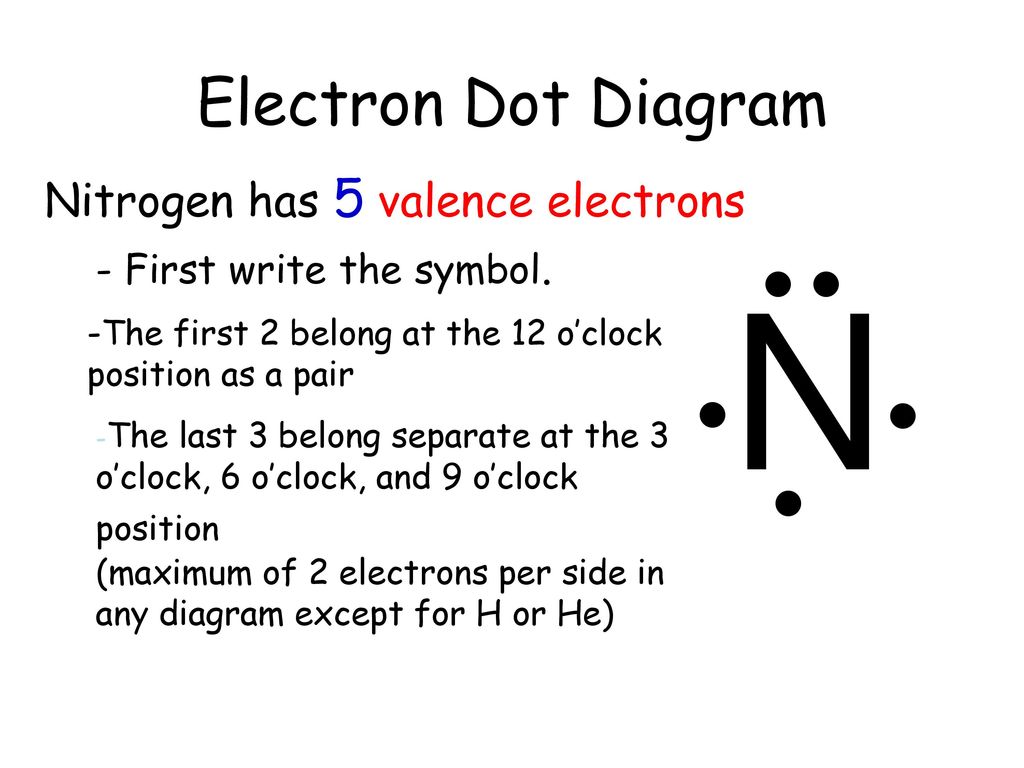 Source: slideplayer.com
Source: slideplayer.com
I show you where Nitrogen is on the periodic table and how to determine. How to draw a dot and cross diagram for ammonia. If you are looking at a nitrogen atom by itself it will have 5 electrons dots surrounding it. Write symbols for the ions formed by the following Gain of 1 electron by Chlorine Gain of 3 electrons by Arsenic Loss of 3 electrons by Iron Loss of 2 electron by Cadmium 4-3. Instead we need to imagine a square around the chemical symbol.
 Source: learnwithdrscott.com
Source: learnwithdrscott.com
The representation of molecules in Lewis electron dot structure or just a Lewis structure is in honour of the American chemist Gilbert Newton Lewis. Lewis dot structures also called electron dot structures are diagrams that describe the chemical bonding between atoms in a molecule. By sharing the three 2p electrons nitrogen can form three covalent bonds. The nitrogen atom has five electron present in 2s and 2p subshell and these electrons are called valence electrons. The representation of molecules in Lewis electron dot structure or just a Lewis structure is in honour of the American chemist Gilbert Newton Lewis.
 Source: youtube.com
Source: youtube.com
Electron dot structure - valence electrons are represented by dots placed around the chemical symbol. We cannot put the dots anywhere around the symbol. Lewis dot structures also called electron dot structures are diagrams that describe the chemical bonding between atoms in a molecule. Electron configuration of the atom. The nitrogen atom has five electron present in 2s and 2p subshell and these electrons are called valence electrons.
 Source: mcqpoint.com
Source: mcqpoint.com
If you are looking at a nitrogen atom by itself it will have 5 electrons dots surrounding it. For diatomic nitrogen the Lewis-dot structure correctly predicts that there will be a triple bond between nitrogen atoms. This triple bond is very strong. The electron dot diagram uses the symbol of the element to replace the nucleus and inner shell electrons. Jon Formation Element Sodium Nitrogen Aluminum Chlorine Calcium Atomic Electron configuration 1s-2522p63s of atom Electron-dot symbol Nae Loss or gain of electrons Lose 1 e Electron configuration of ion 1s22s22p6 Ionic.
 Source: brainly.com
Source: brainly.com
Try to complete the octet of electrons on all the atoms. For diatomic nitrogen the Lewis-dot structure correctly predicts that there will be a triple bond between nitrogen atoms. Of dots around the Electron-dot symbol of the atom. Starting from left to. We cannot put the dots anywhere around the symbol.
 Source: docbrown.info
Source: docbrown.info
The number of dots corresponds to an atoms location on the Periodic Table. The representation of molecules in Lewis electron dot structure or just a Lewis structure is in honour of the American chemist Gilbert Newton Lewis. By sharing the three 2p electrons nitrogen can form three covalent bonds. We represent an electron dot structure by using the symbol of an element. Write electron dot symbols for the following atoms.
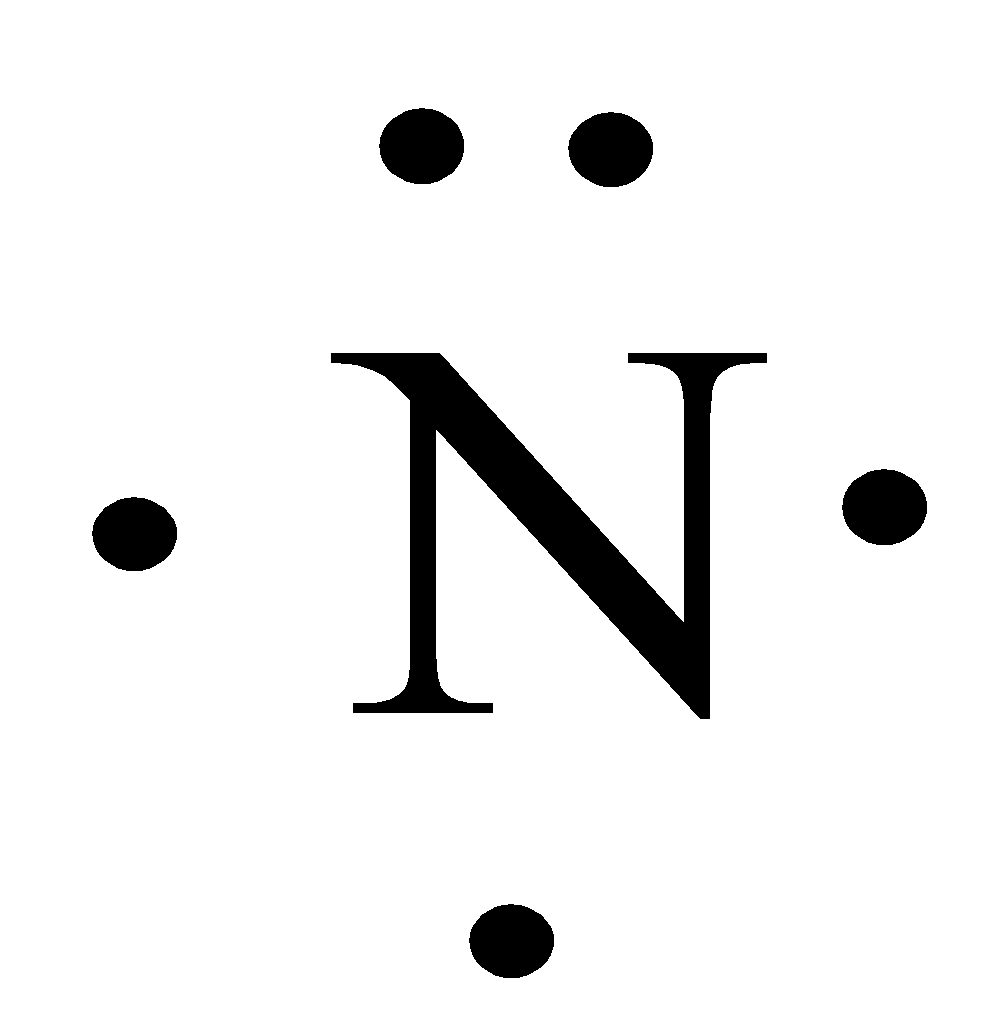 Source: vedantu.com
Source: vedantu.com
Nitrogen dot electron lewis diagram electrons introductory chemistry which oxygen diagrams symbol dots four. Why nitrogen can form only 4 bonds. Lewis dot structures also called electron dot structures are diagrams that describe the chemical bonding between atoms in a molecule. How to draw a dot and cross diagram for ammonia. We represent an electron dot structure by using the symbol of an element.
 Source: youtube.com
Source: youtube.com
Lewis Electron Dot Structure for the molecule. By sharing the three 2p electrons nitrogen can form three covalent bonds. The dots should be neatly drawn on the four sides of the. Symbol including charge of the ion. How to draw a dot and cross diagram for ammonia.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site serviceableness, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title nitrogen electron dot symbol by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






