Your Symbolic notation for isotopes images are ready in this website. Symbolic notation for isotopes are a topic that is being searched for and liked by netizens today. You can Find and Download the Symbolic notation for isotopes files here. Download all royalty-free photos.
If you’re looking for symbolic notation for isotopes pictures information linked to the symbolic notation for isotopes keyword, you have visit the ideal site. Our site frequently provides you with suggestions for viewing the highest quality video and image content, please kindly surf and find more enlightening video content and graphics that match your interests.
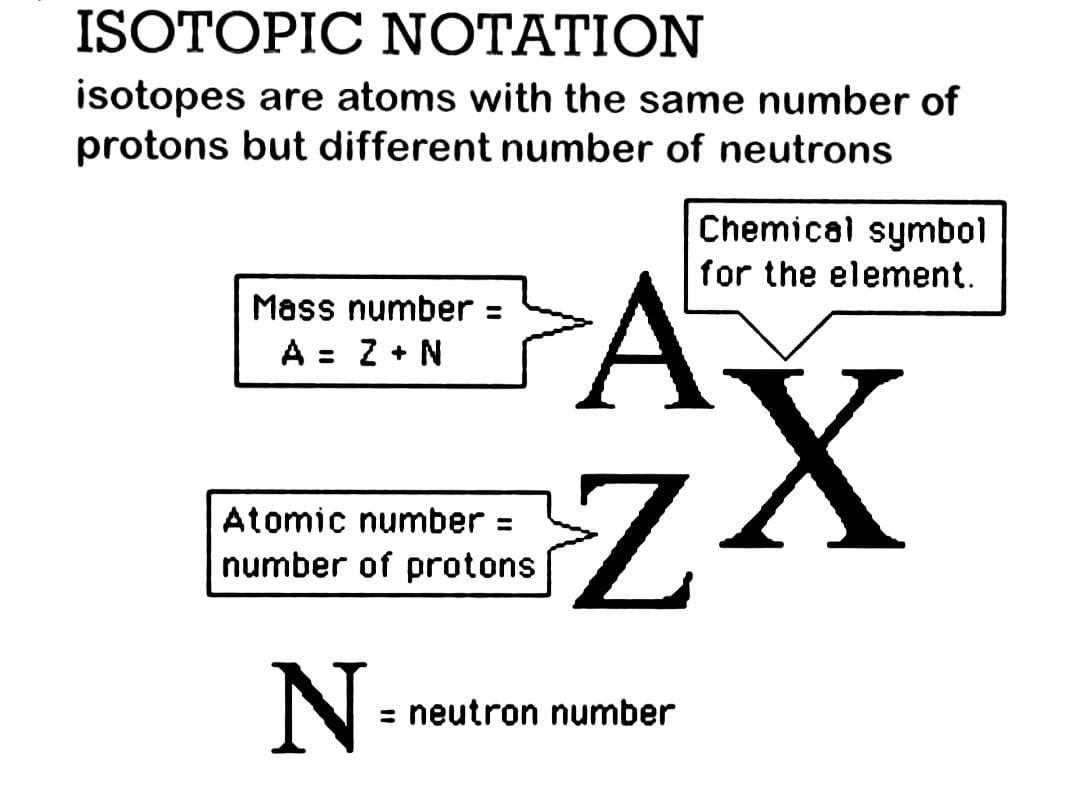
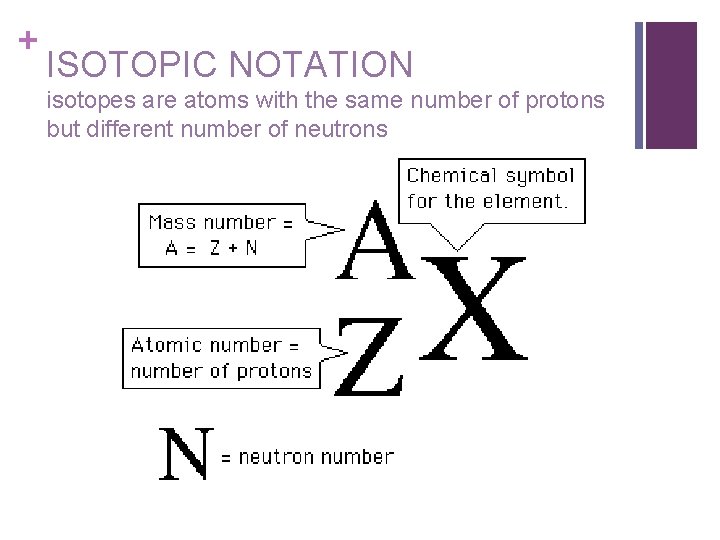
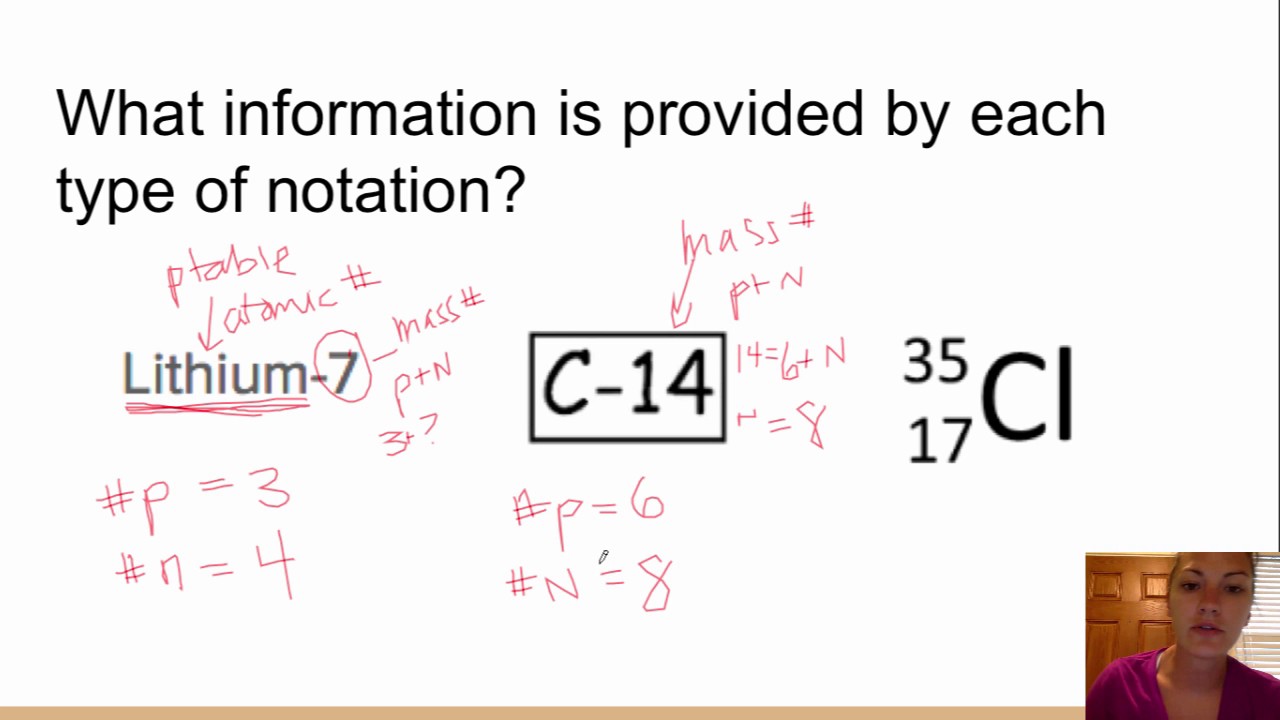
Symbolic Notation For Isotopes. Atomic number mass number. We can use a hypothetical element X to write its symbol. This preview shows page 7 - 8 out of 8 pages. The diagram below gives a general description about how to write the nuclear notation of an isotope of an element correctly.
 Isotope Basics Nidc National Isotope Development Center From isotopes.gov
Isotope Basics Nidc National Isotope Development Center From isotopes.gov
Write the symbolic notation for the following radioactive isotopes 3 points a from CHEM 115 at Ivy Tech Community College of Indiana. We have to write the symbol of an isotope with the given information. Isotopes are written with the name followed by a hyphen and the mass number. Start a live quiz. Isotope notation also known as nuclear notation is important because it allows us to use a visual symbol to easily determine an isotopes mass number atomic number and to determine the number of neutrons and protons in the nucleus without having to. Different isotopes of an element arise from atoms with differing numbers of neutrons.
Isotopes differ in the number of neutrons and therefore their mass numbers.
Instead you have to figure it out based on the number of protons or atomic number. Course Title CHEMISTRY 1. 4019K would represent potassium-40 which is. Isotopes differ in the number of neutrons and therefore their mass numbers. Pages 4 This preview shows page 3 - 4 out of 4 pages. We can use a hypothetical element X to write its symbol.
 Source: makethebrainhappy.com
Source: makethebrainhappy.com
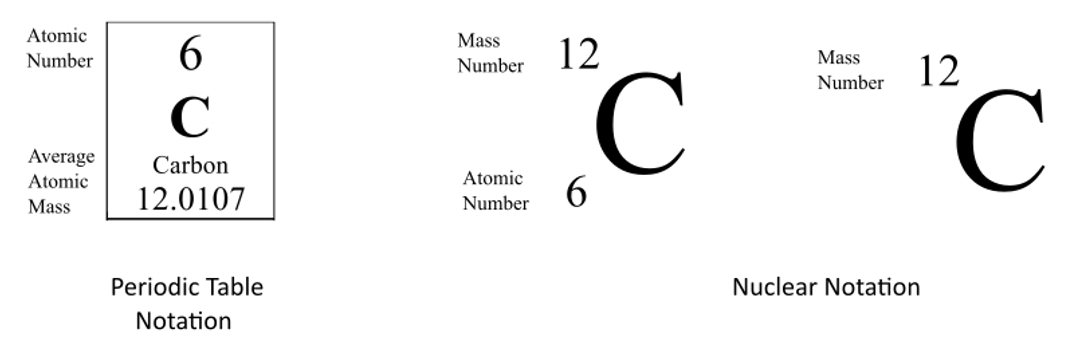
How do you write symbolic notation for an isotope. However most often the best notation is just the O with the superscript of the isotopes mass. Average atomic masses from natural abundances. Along with a Periodic Table that is enough information to tell you that you have. We can use a hypothetical element X to write its symbol.
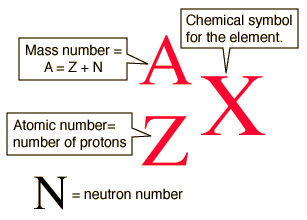 Source: socratic.org
Source: socratic.org
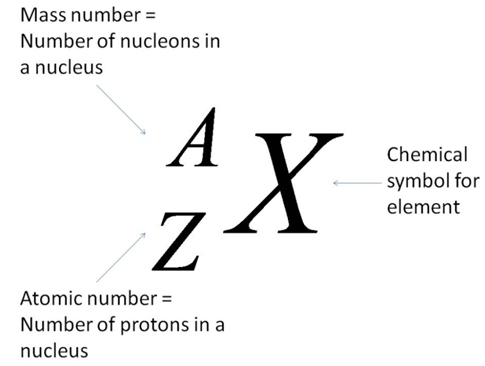
To write the symbol for an isotope place the atomic number as a subscript and the mass number protons plus neutrons as a superscript to the left of the atomic symbol. The nuclear symbol of an isotope indicates the number of protons and neutrons in an atom of the element. It does not indicate the number of electrons. To write the symbol for an isotope place the atomic number as a subscript and the mass number protons plus neutrons as a superscript to the left of the atomic symbol. Nuclear notation contains the elements symbol X along with the mass number A or the atomic number Z or both A and Z.

Average atomic masses from natural abundances. The symbolic notation for these two isotopes would be for carbon 12 with an. Students progress at their own pace and you see a leaderboard and live results. Atomic number mass number. School Georgia Military College Valdosta Campus.
 Source: slideplayer.com
Source: slideplayer.com
Therefore 3919K represents potassium-39 which is potassiums most abundant isotope. What is an isotope notation. Along with a Periodic Table that is enough information to tell you that you have. Isotopes are written with the name followed by a hyphen and the mass number. Start a live quiz.
 Source: slideplayer.com
Source: slideplayer.com
The symbols for the two naturally occurring isotopes of chlorine are written as follows. However most often the best notation is just the O with the superscript of the isotopes mass. In isotope notation you can quickly show. Atomic number mass number. Where A mass number Z atomic number 80 324 ratings View Complete Written Solution Problem Details Write the symbolic notation of an isotope of an element having 8 protons 8 electrons and 11 neutrons.
 Source: terpconnect.umd.edu
Source: terpconnect.umd.edu
The symbolic notation for these two isotopes would be for carbon 12 with an. Students progress at their own pace and you see a leaderboard and live results. To write the symbol for an isotope place the atomic number as a subscript and the mass number protons plus neutrons as a superscript to the left of the atomic symbol. Isotopes and Symbolic Notation DRAFT. The symbolic notation for these two isotopes would be for carbon 12 with an.
 Source: vedantu.com
Source: vedantu.com
Control the pace so everyone advances. In isotope notation you can quickly show. Isotopes and Symbolic Notation DRAFT. We have to write the symbol of an isotope with the given information. Therefore 3919K represents potassium-39 which is potassiums most abundant isotope.
 Source: youtube.com
Source: youtube.com
It does not indicate the number of electrons. A what is the symbolic notation for this isotope b is. This preview shows page 7 - 8 out of 8 pages. However most often the best notation is just the O with the superscript of the isotopes mass. Subscripts and superscripts can be added to an elements symbol to specify a particular isotope of the element and provide other important information.
 Source: slidetodoc.com
Source: slidetodoc.com
Students progress at their own pace and you see a leaderboard and live results. Start a live quiz. Multiply the Mass of each isotope times the Percent. To write the symbol for an isotope place the atomic number as a subscript and the mass number protons plus neutrons as a superscript to the left of the atomic symbol. It does not indicate the number of electrons.
 Source: youtube.com
Source: youtube.com
In isotope notation you can. The symbols for the two naturally occurring isotopes of chlorine are written as follows. Isotope notation also known as nuclear notation is important because it allows us to use a visual symbol to easily determine an isotopes mass number atomic number and to determine the number of neutrons and protons in the nucleus without having to. We have to write the symbol of an isotope with the given information. Control the pace so.
 Source: cpanhd.sitehost.iu.edu
Source: cpanhd.sitehost.iu.edu
The symbols for the two naturally occurring isotopes of chlorine are written as follows. Exp 10 A Greener Bromination of Stilbene. The number of neutrons is not stated. 2 days ago by. What is an isotope notation.
 Source: ibslandhlchemistry.blogspot.com
Source: ibslandhlchemistry.blogspot.com
Control the pace so everyone advances. How do you write isotopes in symbolic notation. Lithium has two isotopes. The symbols for the two naturally occurring isotopes of chlorine are written as follows. Naming and Notation Intro to Isotopes A N Z Calcs Abundance Notation Naming Isotopes vs Ions Uses Stability More Chem Help Notation and Naming AZE notation In chemistry naming and notation are essential for clear communication.
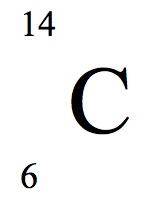 Source: ehschemcorner.blogspot.com
Source: ehschemcorner.blogspot.com
It does not indicate the number of electrons. Students who viewed this also studied. Write the symbolic notation of on isotope of an element having 8 protons 8 electrons and 8 neutrons. School Georgia Military College Valdosta Campus. What is an isotope notation.

The nuclear symbol of an isotope indicates the number of protons and neutrons in an atom of the element. Isotope notation also known as nuclear notation is important because it allows us to use a visual symbol to easily determine an isotopes mass number atomic number and to determine the number of neutrons and protons in the nucleus without having to. We have to write the symbol of an isotope with the given information. Average atomic masses from natural abundances. The diagram below gives a general description about how to write the nuclear notation of an isotope of an element correctly.
 Source: studylib.net
Source: studylib.net
Nuclear notation contains the elements symbol X along with the mass number A or the atomic number Z or both A and Z. It does not indicate the number of electrons. Average atomic masses from natural abundances. Lithium has two isotopes. Start a live quiz.
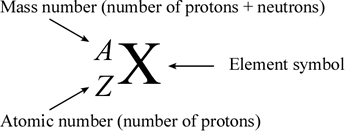 Source: guweb2.gonzaga.edu
Source: guweb2.gonzaga.edu
To write the symbol for an isotope place the atomic number as a subscript and the mass number protons plus neutrons as a superscript to the left of the atomic symbol. The symbolic notation for these two isotopes would be. In isotope notation you can. Along with a Periodic Table that is enough information to tell you that you have. Isotope notation also known as nuclear notation is important because it allows us to use a visual symbol to easily determine an isotopes mass number atomic number and to determine the number of neutrons and protons in the nucleus without having to use a lot of words.
 Source: quizlet.com
Source: quizlet.com
An hour ago by. Subscripts and superscripts can be added to an elements symbol to specify a particular isotope of the element and provide other important information. Pages 8 Ratings 75 4 3 out of 4 people found this document helpful. This preview shows page 7 - 8 out of 8 pages. Instead you have to figure it out based on the number of protons or atomic number.
 Source: slideplayer.com
Source: slideplayer.com
How do you write isotopes in symbolic notation. Label the protons electrons neutrons. However most often the best notation is just the O with the superscript of the isotopes mass. It does not indicate the number of electrons. Isotope notation also known as nuclear notation is important because it allows us to use a visual symbol to easily determine an isotopes mass number atomic number and to determine the number of neutrons and protons in the nucleus without having to use a lot of words.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site value, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title symbolic notation for isotopes by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






